The use of H O in high-temperature corrosion studies

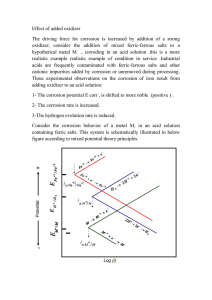
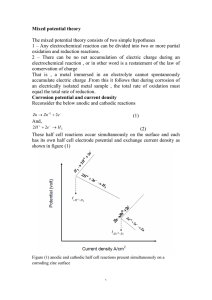
The use of H
2
18
O in high-temperature corrosion studies
Juho Lehmusto, Jingxin Sui, Mikael Bergelin, Patrik Yrjas
Laboratory of Inorganic Chemistry
Johan Gadolin Process Chemistry Centre
Abo Akademi University
In boilers firing biomass and waste-derived fuels, uncontrolled and detrimental oxidation of the heat-transfer materials is one of the biggest obstacles in increasing power production efficiency. The main cause of material degradation is the prevailing chemical environment, which contains, among others, water vapor and corrosive species such as potassium chloride.
In order to study the effect of water vapor in greater detail in the potassium chlorideaccelerated oxidation process, oxygen isotope
18
O is used to clarify the reaction mechanism of high-temperature corrosion in the case with multiple oxygen sources. For the identification of oxygen isotope
18
O, Time-of-Flight Secondary Ion Mass Spectrometry (ToF SIMS), an analysis method rarely used in high-temperature corrosion studies, will be used.
The main goals of the study are:
1) to evaluate the applicability of ToF-SIMS in high-temperature corrosion studies;
2) to shed more light on the oxidation behavior of various steels under conditions, where multiple oxygen sources are present