UTSW Alzheimer’s Disease Center (ADC) Data Request Form
advertisement

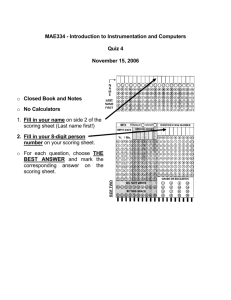
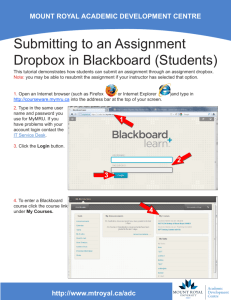
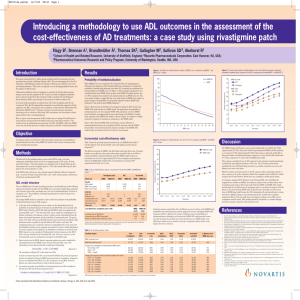
UTSW Alzheimer’s Disease Center (ADC) Data Request Form 1. Complete this form and submit to: Roger N. Rosenberg, M.D., Director - ADC Department of Neurology and Neurotherapeutics University of Texas Southwestern Medical Center 5323 Harry Hines Boulevard, MC: 9036 Dallas, TX 75390-9036 2. The ADC Committee will consider your request. You will be notified regarding the Committee's action. 3. In an effort to provide optimal support for your projects, please briefly describe the data below that you are requesting from the ADC database or that you propose to acquire from our subjects. If you are unsure, please indicate that as well, so we can discuss this with you. Investigator: Department: Date : Summarize your project and the data you are requesting: Diagnoses of interest: AD MCI (what type) FTD DLB Normal Controls Other _____ What demographics are Age Gender Other (specify) desired? Imaging Imaging Data Desired? Propose using already acquired data Proposing using new scans For new scans, please indicate what sequences you propose. We ________ MPRAGE sense request that the following sequences from our ADC protocol be ________ ADNI high-resolution T1 acquired and deposited in the ADC neuroimaging database as part of ________ T2 sense your study. Any omissions will need justification. Our standard 3T ________ T2 FLAIR MRI protocol consists of the following sequences: ________ DTI (32 directions, 1.75x1.75x2 with 1mm gap) sense ________ fcMRI ________ minutes Please list any additional sequences (and acquisition times) needed for your specific study: Biomarkers Data already in database Frozen samples for new assays New sample collections to assay Blood Yes No CSF Yes No Neuropsychology What global measures are wanted (e.g., CERAD total score, MMSE, ______________________________________________________ etc.)? Specific neuropsychological tests (please see Appendix 1) or cognitive ______________________________________________________ domains? Are there additional tests that you plan to administer? ______________________________________________________ Need to discuss/consult with a neuropsychologist? Yes No Neuropsychiatric and global function variables Mood (Geriatric Depression Scale) Yes No Specify: __________________________________ Behavior: (NPI) Yes No Specify: __________________________________ Function: (CDR, FAQ, TFLS, etc) Yes No Specify: __________________________________ Clinical variables Physical exam variables: _______________________________________________________ UPDRS motor sub-scale: _______________________________________________________ Clinical history variables: _______________________________________________________ Other Comments or requests: Please include any other comments or requests: Please indicate the tests of interest and desired data: Please be sure to credit the ADC grant (NIH P30-AG12300) when submitting grant applications and manuscripts. Review Status:_________________________________________________________________________ Appendix 1 Neuropsychological Testing Protocols Neuropsychological data is collected by participant cohort according to the following testing protocols: Normal Controls / Mild Cognitive Impairment: - Mini Mental State Examination (MMSE) Logical Memory – Story A Digit Span Category Fluency Trail Making Test Digit Symbol Coding Boston Naming Test Visual Reproduction (if MMSE > 23) California Verbal Learning Test (if MMSE > 23) Block Design Letter Fluency (FAS) Wisconsin Card Sorting Test (if Trails B < 300 sec.) CERAD Geriatric Depression Scale Alzheimer’s Disease / Lewy Body Dementia - Mini Mental State Examination (MMSE) Logical Memory – Story A Digit Span Category Fluency Trail Making Test Digit Symbol Coding Boston Naming Test Visual Reproduction (if MMSE > 23) California Verbal Learning Test (if MMSE > 23) Letter Fluency (FAS) Wisconsin Card Sorting Test (if Trails B < 300 sec.) AMNART CERAD Geriatric Depression Scale