2. Fundamental Types of Lattices – cont. a ,
advertisement
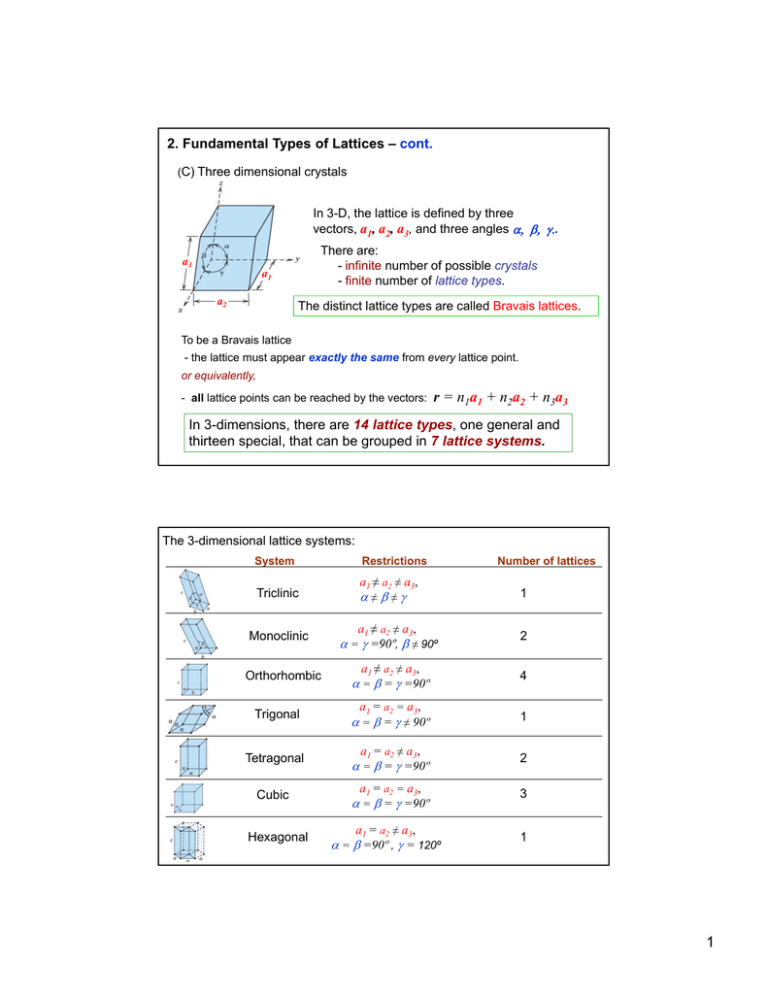
2. Fundamental Types of Lattices – cont.
(C) Three dimensional crystals
In 3-D, the lattice is defined by three
vectors, a1, a2, a3, and three angles .
There are:
- infinite number of possible crystals
- finite number of lattice types.
a3
a1
a2
The distinct lattice types are called Bravais lattices.
To be a Bravais lattice
- the lattice must appear exactly the same from every lattice point.
or equivalently,
- all lattice points can be reached by the vectors:
r = n1a1 + n2a2 + n3a3
In 3-dimensions, there are 14 lattice types, one general and
thirteen special, that can be grouped in 7 lattice systems.
The 3-dimensional lattice systems:
System
Restrictions
Triclinic
≠≠
1
a1 ≠ a2 ≠ a3,
2
a1 ≠ a2 ≠ a3,
4
Monoclinic
Orthorhombic
a1 ≠ a2 ≠ a3,
= =90º,
90º ≠ 90º
= = =90º
a1 = a2 = a3,
Number of lattices
Trigonal
= = ≠ 90º
1
Tetragonal
= = =90º
a1 = a2 ≠ a3,
2
a1 = a2 = a3,
3
Cubic
Hexagonal
= = =90º
a1 = a2 ≠ a3,
= =90º , = 120º
1
1
The 3-D Bravais lattices (a = a1 , b= a2 , c=a3 )
Cubic lattices:
Simple Cubic (SC). Example: Po
Body Centered Cubic (BCC). Examples: Fe, Na, Li
Face Centered
C
C
Cubic ((FCC).
CC) Examples: C
Cu, Ag, Au, Ni, Pd, Pt, Al
2
BCC and FCC cells are conventional unit cells, not primitive
cells, but BCC and FCC lattices are Bravais lattices.
The Cubic lattice unit cells can be shown by lattice point positions
a
a
a
0
0
1
0
1
1
0
1
½
1/2
0
½
0
1
0
0
½
1
1
½
01
1
Geometric properties of the Cubic lattice system
Atomic Packing Fraction
Volume of atoms in the cell
=
Volume of the unit cell
a
aa
aa
a
0
0
1
½
0
1
1
Simple Cubic
(SC)
Conventional cell Volume
Lattice points per cell
Primitive cell volume
# of nearest neighbors
Nearest neighbor distance
Packing fraction
a3
1
a3
6
a
0.524
0
1
1
1/2
0
½
0
0
1
½
1
½
01
0
1
Body Centered Face Centered
Cubic (BCC)
Cubic (FCC)
a3
2
a3/2
8
a3
4
a3/4
12
0.866a
0.680
0.707a
0.704
3
Primitive cells for BCC and FCC
The SC is a primitive cell, but the BCC and FCC are conventional unit cells. A
conventional cell (non-primitive) often has a more obvious relation with the symmetry
operations. Primitive cells of BCC and FCC lattices can be chosen in various ways.
z
Primitive cell
BCC lattice
One method to
a3
choose primitive
a
a1
translation vectors
a2
x
Primitive lattice vectors a1 axˆ;
y
a2 ayˆ ;
1
a3 a ( xˆ yˆ zˆ )
2
A more symmetric choice:
1
3a
2
z
a3
x
a2
a1
109 28'
y
Primitive cell
Primitive lattice vectors
1
1
1
a1 a ( xˆ yˆ zˆ ); a2 a ( xˆ yˆ zˆ ); a3 a ( xˆ yˆ zˆ )
2
2
2
The Wigner-Seitz primitive cell for BCC
FCC lattice
Primitive lattice vectors
1
1
1
a1 a ( xˆ yˆ ); a2 a ( yˆ zˆ ); a3 a ( zˆ xˆ )
2
2
2
4
The Wigner-Seitz primitive cell for FCC
Primitive cells for hexagonal system
a1 = a2 = a
a3 = c
a3
a3
a2
a2
a1
a1
3. Index System for Crystal Planes (Miller Indices)
In a crystal lattice, there are sets of parallel planes. The distances between
adjacent lattice planes yield distinct X-ray diffraction patterns for structure
determination.
A plane set can be described by
3 indices hkl (Miller indices).
For example:
( 1 0 0)
(1 1 0)
(1 1 1)
5
Procedure to determine the Miller indices of a plane:
(1) Find the intercepts of the plane with the three
lattice axes: n1a1, n2a2 and n3a3, where
are either integers or rational fractions
a3
ni
n 3a 3
(2) Find 1/n1, 1/n2 and 1/n3.
n 2a 2 a
2
(3) Reduce (1/n
1/ 1,1/n
1/ 2,1/n
1/ 3) to three integers
(h,k,l) with the same ratio. The set of number (h
k l) are the Miller indices of the plane.
a1
n 1a 1
Example: Find the Miller indices of
the planes A, B and C.
A: (1)
C
a
n1=1, n2=∞, n3=∞
a
(2) 1/n1=1, 1/n2=0, 1/n3=0
(3) The Miller indices are (1, 0, 0).
B
A
a
Plane A is (100) plane.
Example: Find the Miller indices
of the planes A and B.
S
Some
common planes
l
z
y
x
SC lattice
100 planes
110 planes
111 planes
FCC lattice
200 planes
220 planes
111 planes
BCC lattice
200 planes
110 planes
222 planes
6
Planes equivalent by symmetry are denoted as {hkl}, which represents a family
of planes.
For example, {100} family of a cubic lattice includes all the following planes
(100)
(001)
(010)
z
z
z
y
x
y
y
x
x
(100)
(010)
(001)
Directions in a crystal lattice are represented by [uvw].
To find the indices of a direction:
(1) Find the components of the direction vector: n1a1, n2a2 and n3a3.
(2) Reduce n1, n2 and n3 to a set of smallest integers, uvw, with the same ratio.
For example:
A: [1 1 0];
B: [1 2 1];
C: [2 0 1];
D: [1 2 1];
7
4. Simple Crystal Structures
(A) NaCl structure
Lattice: FCC
Basis: 1 Na+ and 1 Cl- ion.
There are 4 formula units
(NaCl) in a unit cell.
(B) CsCl Structure
Lattice: SC
Basis: 1 Cs+ and 1 Cl- ion.
There is one formula unit (CsCl) in
a unit cell.
(C) Hexagonal Close-packed (HCP) Structure
FCC
A layer
B layer
C layer
a
A layer
A layer
A layer
B layer
B layer
A layer
HCP
Lattice: HCP (Be, Mg)
Basis: 2 atoms (000, 23 13 21)
PF =0.74
8





