Physics 531 Fall 2004 D. Sober The ingredients of the derivation are
advertisement
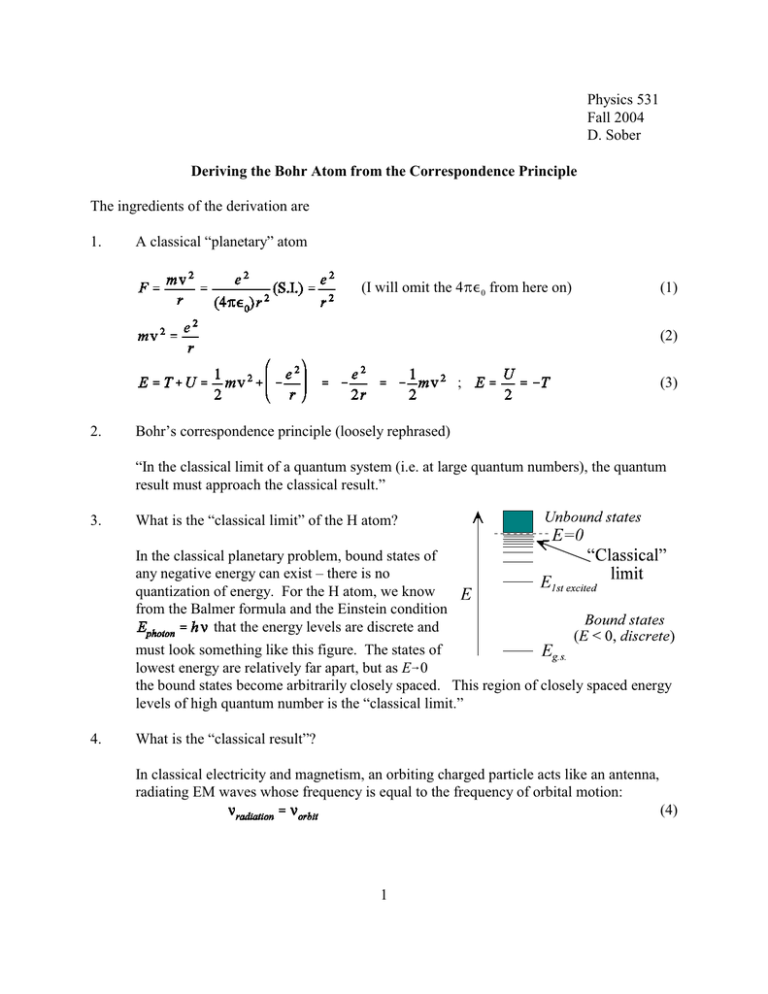
Physics 531 Fall 2004 D. Sober Deriving the Bohr Atom from the Correspondence Principle The ingredients of the derivation are 1. A classical “planetary” atom (I will omit the 4B,0 from here on) (1) (2) ; 2. (3) Bohr’s correspondence principle (loosely rephrased) “In the classical limit of a quantum system (i.e. at large quantum numbers), the quantum result must approach the classical result.” 3. What is the “classical limit” of the H atom? In the classical planetary problem, bound states of any negative energy can exist – there is no quantization of energy. For the H atom, we know from the Balmer formula and the Einstein condition that the energy levels are discrete and must look something like this figure. The states of lowest energy are relatively far apart, but as E60 the bound states become arbitrarily closely spaced. This region of closely spaced energy levels of high quantum number is the “classical limit.” 4. What is the “classical result”? In classical electricity and magnetism, an orbiting charged particle acts like an antenna, radiating EM waves whose frequency is equal to the frequency of orbital motion: (4) 1 5. Equate the classical and quantum results in the classical limit: Consider a transition between two adjacent energy levels in the highly excited region. By energy conservation, the EM energy emitted is equal to the energy difference )E between the two states. But according to Einstein’s formula, this EM energy takes the form of a photon of energy , (5) where <radiation is the frequency of the EM radiation. Combining (4) and (5) we obtain . (6) 6. Derive a quantization condition to determine the allowed orbits To convert this to a quantization condition for the atomic orbits, we must express the energy E in some appropriate way. In equation (3), we see that E can be written in terms of either the orbit radius r or the orbit velocity v . Another possibility is to write it in terms of the classical angular momentum l=mvr. (7) Using (2) and (7), we obtain , , and , (8) leading to . (9) Now note that as we approach the classical limit, *E*6 0 , v 6 0, r 64, and l 64 , so lupper and llower are large numbers differing by only a small fraction of their values. Thus we can use the first term of a Taylor expansion and write . (10) Combining (9) and (10) we obtain . (11) Now use (6), (11) and (2): , 2 (12) i.e. adjacent states have values of angular momentum which differ by S (at least in the limit of large l). Extending this result to all l, l = nS + constant = nS , n = 1, 2, 3,..., (13) where the constant must be 0 to agree with the Balmer formula (Eq. (15).) Thus the quantization condition for the Bohr atom, equation (13), can be derived from classical mechanics and the correspondence principle. The only specifically quantum input to the calculation is (5), Einstein’s photon hypothesis. Using the (incorrect) quantization of angular momentum given by (13), the energy levels of the H atom are given by (8): (14) where R4 is the Rydberg constant (for infinite nuclear mass): , . (15) The energy levels given by the Bohr model agree with those derived from the Schrödinger equation, which is a remarkable result when one considers that almost everything about the Bohr model is incorrect – including the crucial quantization rule for angular momentum, Eq. (13). This coincidence is partly explained by considerations of dimensional analysis: we will soon show that the energy levels of the H atom must be given by (m e4 / h2) multiplied by dimensionless quantities. The fact that the Bohr model gives the quantitatively correct energy spectrum even far from the classical limit must be viewed as an accident – a fortunate one for the history of quantum physics. The Bohr model also gives – with slightly skewed interpretations – two important additional numerical constants of quantum mechanics. The radius of the nth Bohr orbit is where . (In the quantum-mechanical solution, a0 appears as a length scale parameter in the radial part of the wave function.) The velocity of the electron in the nth Bohr orbit is vn = e2/nS. In the ground . The square of " state (n=1), gives the order of magnitude of the relativistic and spin-orbit corrections which appear in the fine structure of the H atom. 3








