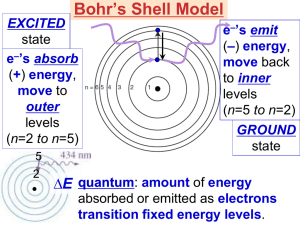
7. QUANTUM THEORY OF THE ATOM Solutions to Practice Problems
advertisement
7. QUANTUM THEORY OF THE ATOM Solutions to Practice Problems Note on significant figures: If the final answer to a solution needs to be rounded off, it is given first with one nonsignificant figure, and the last significant figure is underlined. The final answer is then rounded to the correct number of significant figures. In multiple-step problems, intermediate answers are given with at least one nonsignificant figure; however, only the final answer has been rounded off. Starting with Problem 7.29, the value 2.998 x 108 m/s will be used for the speed of light. 7.29 Solve c = for : = 7.30 Solve c = for : = 7.31 2.998 x 108 m/s c = = 6.271 x 1014 = 6.27 x 1014 /s 7 4.78 x 10 m Solve c = for . Recognize that 656 nm = 656 x 10-9 m = 6.56 x 10-7 m. = 7.33 c 2.998 x 108 m/s = = 0.023831 = 0.02383 m (2.383 cm) 1.258 x 1010 /s Solve c = for . Recognize that 478 nm = 478 x 10-9 m, or 4.78 x 10-7 m. = 7.32 c 2.998 x 108 m/s = = 219.63 = 219.6 m 1.365 x 10 6 /s 2.998 x 108 m/s c = = 4.570 x 1014 = 4.57 x 1014 /s 6.56 x 10 -7 m Radio waves travel at the speed of light, so divide the distance by c: 56 x 109 m x 7.34 1s 2.998 x 108 m = 186.7 = 1.9 x 102 s Electromagnetic signals travel at the speed of light, so divide the distance by c: QUANTUM THEORY OF THE ATOM 247 998 x 109 m x 1s 8 = 3328.8 = 3.33 x 103 s (0.925 hr) 2.998 x 10 m 7.35 7.36 To do the calculation, divide one meter by the number of wavelengths in one meter to find the wavelength of this transition. Then, use the speed of light (with nine digits for significant figures) to calculate the frequency: = 1m = 6.507802106 x 10-7 m 1,650,763.73 = 2.99792458x 108 m/ s c = 4.948865162 x 1014 = 4.94886516 x 1014 /s = -7 6.507802106 x 10 m To find the wavelength, divide the speed of light (with nine digits for significant figures) by the frequency: = 7.37 2.99792458x 108 m/ s c = 0.03261225572 = 0.0326122557 m = 9,192,631,770 /s Solve for E, using E = h, and use four significant figures for h: E = h = (6.626 x 10-34 Js) x (1.365 x 106 /s) = 9.0444 x 10-28 = 9.044 x 10-28 J 7.38 Solve for E, using E = h, and use four significant figures for h: E = h = (6.626 x 10-34 Js) x (1.258 x 1010 /s) = 8.33550 x 10-24 = 8.336 x 10-24 J 7.39 Recognize that 535 nm = 535 x 10-9 m = 5.35 x 10-7 m. Then, calculate and E. = c 2.998 x 108 m/s = = 5.6037 x 1014 /s 7 5.35 x 10 m E = h = (6.626 x 10-34 Js) x (5.6037 x 1014/s) = 3.713 x 10-19 = 3.71 x 10-19 J 7.40 Recognize that 451 nm = 451 x 10-9 m = 4.51 x 10-7 m. Then, calculate and E. = c 2.998 x 108 m/s = = 6.6474 x 1014 /s 7 4.51x 10 m E = h = (6.626 x 10-34 Js) x (6.6474 x 1014/s) = 4.404 x 10-19 = 4.40 x 10-19 J 248 CHAPTER 7 7.41 First, calculate the wavelength of this transition from the frequency using the speed of light: = c 2.998 x 108 m/s = = 7.8072 x 10-7 = 7.81 x 10-7 m (781 pm) 3.84 x 1014 /s Using Figure 7.5, note that 781 nm is just on the edge of the red end of the spectrum and is barely visible to the eye. 7.42 First, calculate the wavelength of this transition from the frequency using the speed of light: = 2.998 x 108 m/s c = = 5.541 x 10-7 = 5.54 x 10-7 m (554 pm) 5.41x 1014 /s Using Figure 7.5, note that 554 nm is in the yellow-green region of the spectrum and is visible to the eye. 7.43 Solve the equation E = -RH/n2 for both E5 and E3; equate to h, and solve for . E5 = - RH 5 2 = - RH ; 25 E3 = - RH 3 2 = - RH 9 16 RH - RH - RH = h - = 225 25 9 The frequency of the emitted radiation is: = 7.44 16 R H 225 h = 16 2.179 x 10 -18 J x = 2.338 x 1014 = 2.34 x 1014 /s -34 225 6.626 x 10 J s Solve the equation E = -RH/n2 for both E4 and E3; equate to h, and solve for . E4 = - RH 4 2 = - RH ; 16 E3 = - RH 3 2 = - RH 9 7 RH - RH - RH = h - = 144 16 9 The frequency of the emitted radiation is: = 7.45 7 RH 144 h = 7 2.179 x 10 -18 J x = 1.5986 x 1014 = 1.60 x 1014 /s -34 144 6.626 x 10 J s Solve the equation E = -RH/n2 for both E2 and E1. E2 = - RH 2 2 = - RH ; 4 E1 = - RH 2 1 = - RH 1 QUANTUM THEORY OF THE ATOM 249 3 RH - RH - RH = h - = 4 4 1 The frequency of the emitted radiation is: = 3 RH 4h = 2.179 x 10 -18 J 3 x = 2.466 x 1015 /s 4 6.626 x 10 -34 J s The wavelength can now be calculated. = 7.46 c 2.998 x 108 m/s = = 1.2155 x 10-7 = 1.22 x 10-7 m (near UV) 15 2.466 x 10 /s Solve the equation E = -RH/n2 for both E5 and E4; solve for , and convert to . E5 = - RH 5 2 = - RH ; 25 E4 = - RH - RH = 2 16 4 9 RH - RH - RH = h - = 400 25 16 The frequency of the emitted radiation is: = 9 RH 400 h = 9 2.179 x 10 -18 J x = 7.399 x 1013 /s -34 400 6.626 x 10 J s The wavelength can now be calculated. = c 2.998 x 108 m/s = = 4.0517 x 10-6 = 4.05 x 10-6 m (near IR) 13 7.399 x 10 /s 250 CHAPTER 7 7.47 This is the highest energy transition from the n = 6 level, so the electron must undergo a transition to the n = 1 level. Solve the Balmer equation using Bohr's approach: E6 = - RH 6 2 = - RH ; 36 E1 = - RH 2 1 = - RH 1 35 RH - R - R h = H - H = 36 36 1 The frequency of the emitted radiation is: = 35 R H 36 h = 2.179 x 10 -18 J 35 x = 3.197 x 1015 /s 36 6.626 x 10 -34 J s The wavelength can now be calculated. = 7.48 c 2.998 x 108 m/s = = 9.3769 x 10-8 = 9.38 x 10-8 m (93.8 nm) 3.197 x 1015 /s This is the lowest energy transition from the n = 6 level, so the electron must undergo a transition to the n = 5 level. Solve the Balmer equation using Bohr's approach: E6 = - RH 6 2 = - RH ; 36 E5 = - RH - RH = 2 25 5 11RH - R - R h = H - H = 900 36 25 = = 11R H 900 h = 11 2.179 x 10 -18 J x = 4.019 x 1013 /s -34 900 6.626 x 10 J s c 2.998 x 108 m/s = = 7.458 x 10-6 = 7.46 x 10-6 m (7460 nm) 13 4.019 x 10 /s QUANTUM THEORY OF THE ATOM 251 7.49 Noting that 422.7 nm = 4.227 x 10-7 m, convert the 422.7 nm to frequency. Then convert the frequency to energy using E = h. = c 2.998 x 108 m/s = = 7.0925 x 1014 /s -7 4.227 x 10 m E = h = (6.626 x 10-34 Js) x 7.0925 x 1014 /s) = 4.6994 x 10-19 = 4.699 x 10-19 J 7.50 Noting that 285.2 nm = 2.852 x 10-7 m, convert the 285.2-nm wavelength to frequency. Then convert the frequency to energy using E = h. = 2.998 x 108 m/s c = = 1.0511 x 1015 /s 2.852 x 10 -7 m E = h = (6.626 x 10-34 Js) x 1.0511 x 1015 /s) = 6.96458 x 10-19 = 6.965 x 10-19 J 7.51 The mass of a neutron = 1.67493 x 10-27 kg. Its speed or velocity, v, of 4.15 km/s equals 4.15 x 103 m/s. Substitute these parameters into the de Broglie relation, and solve for : = 6.626 x 10 -34 kg m 2 / s h = mv 1.67493 x 10 -27 kg x 4.15 x 103 m/ s = 9.532 x 10-11 = 9.53 x 10-11 m 7.52 or 95.3 pm The mass of a proton = 1.67262 x 10-27. Its speed or velocity, v, of 6.21 km/s equals 6.21 x 103 m/s. Substitute these parameters into the de Broglie relation, and solve for : = 6.626 x 10 -34 kg m 2 / s h = mv 1.67262 x 10 -27 kg x 6.21x 103 m/ s = 6.379 x 10-11 = 6.38 x 10-11 m or 63.8 pm A wavelength of 63.8 pm would be in the x-ray region of the spectrum. 252 CHAPTER 7 7.53 The mass of an electron equals 9.10953 x 10-31 kg. The wavelength, , given as 10.0 pm, is equivalent to 1.00 x 10-11 m. Substitute these parameters into the de Broglie relation, and solve for the frequency, : v = 6.626 x 10 -34 kg m 2 / s h = = 7.273 x 107 -31 -11 m 9.10953 x 10 kg x 1.00 x 10 m = 7.27 x 107 m/s 7.54 The mass of a neutron equals 1.67493 x 10-27 kg. The wavelength, , given as 10.0 pm, is equivalent to 1.00 x 10-11 m. Substitute these parameters into the de Broglie relation, and solve for the frequency, : v = 6.626 x 10 -34 kg m 2 / s h = = 3.955 x 104 -27 -11 m 1.67493 x 10 kg x 1.00 x 10 m = 3.96 x 104 m/s 7.55 Substitute the 1.45 x 10-1 kg mass of the baseball and the 30.0 m/s velocity, v, into the de Broglie relation, and solve for wavelength (recall that 1 pm = 10-12 m). = h 6.626 x 10-34 kg m2 / s = = 1.523 x 10-34 =1.52 x 10-34 m -1 mv 1.45 x 10 kg x 30.0 m/ s = 1.52 x 10-22 pm Because this is much smaller than 100 pm, the wavelength is much smaller than the diameter of one atom. 7.56 The mass of O2 to three significant figures is 32.00 6.022 x 1023 = 5.313 x 10-23 g = 5.313 x 10-26 kg. Substitute this mass and the 521 m/s velocity, v, into the de Broglie relation, and solve for wavelength (recall that 1 pm = 10-12 m). = 6.626 x 10 -34 kg m 2/ s h = = 2.3937 x 10-11 = 2.39 x 10-11 m 26 mv 5.313 x 10 kg x 521m/s = 23.9 pm Because this is on the order of 100 pm, the wavelength is on the order of an atomic diameter. QUANTUM THEORY OF THE ATOM 253 7.57 The possible values of l range from zero to (n - 1), so l may be 0, 1, 2, or 3. The possible values of ml range from - l to + l, so ml may be -3, -2, -1, 0, +1, +2, or +3. 7.58 The possible l values are 0, 1, 2, 3, and 4. The possible ml values are -4, -3, -2, -1, 0, 1, 2, 3, or 4. 7.59 For the M shell, n = 3; there are three subshells in this shell (l = 0, 1, and 2). An f subshell has l = 3; the number of orbitals in this subshell is 2(3) + 1 = 7 (ml = -3, -2, -1, 0, 1, 2, and 3). 7.60 For the N shell, n = 4; there are four subshells in this shell (l = 0, 1, 2, and 3). For a g subshell, the value of l = 4; the number of orbitals in this subshell is 2(4) + 1 = 9 (ml = -4, -3, -2, -1, 0, 1, 2, 3, and 4). 7.61 a. 6d b. 5g c. 4f d. 6p 7.62 a. 3p b. 4d c. 4s d. 5f 7.63 a. Not permissible; ms may be only + 1/2 or -1/2. b. Not permissible; l can only be as large as (n - 1). c. Not permissible; ml may not exceed +2 in magnitude. d. Not permissible; n may not be zero. e. Not permissible; ms may only be + 1/2 or -1/2. 7.64 a. Not permissible; n starts at 1, not at zero. b. Not permissible; l may only be as large as (n - 1). c. Permissible. d. Not permissible; ml may not exceed l in magnitude. e. Permissible. 254 CHAPTER 7 Solutions to General Problems 7.65 Use c = to calculate frequency; then use E = h to calculate energy. = c 2.998 x 108 m/s = = 6.503 x 1014 = 6.50 x 1014 /s -7 4.61x 10 m E = h = (6.626 x 10-34 Js) x (6.503 x 1014/s) = 4.309 x 10-19 = 4.31 x 10-19 J 7.66 Use c = to calculate frequency; then use E = h to calculate energy. = c 2.998 x 108 m/s = = 5.4115 x 1014 = 5.41 x 1014 /s 7 5.54 x 10 m E = h = (6.626 x 10-34 Js) x (5.4115 x 1014/s) = 3.5856 x 10-19 = 3.59 x 10-19 J 7.67 7.68 Calculate the frequency corresponding to 4.10 x 10-19 J. Then convert that to wavelength. = 4.10 x 10 -19 J E = = 6.187 x 1014 /s -34 h 6.626 x 10 J s = c 2.998 x 108 m/s = = 4.845 x 10-7 = 4.85 x 10-7 m = 485 nm (blue) 14 6.187 x 10 /s Calculate the frequency corresponding to 3.34 x 10-19 J. Then convert that to wavelength. = 3.34 x 10 -19 J E = = 5.0407 x 1014 /s -34 h 6.626 x 10 J s = 2.998 x 108 m/s c = = 5.947 x 10-7 = 5.95 x 10-7 m = 595 nm (yellow) 14 5.0407 x 10 /s QUANTUM THEORY OF THE ATOM 255 7.69 Solve for frequency using E = h. = 7.70 Solve for frequency using E = h. = 7.71 E 4.34 x 10 -19 J = = 6.549 x 1014 = 6.55 x 1014 /s -34 h 6.626 x 10 J s 5.90 x 10 -19 J E = = 8.904 x 1014 = 8.90 x 1014 /s -34 h 6.626 x 10 J s First calculate Ep, the energy of the 345-nm photon, noting that it is equivalent to 3.45 x 10-7 m. Ep = (6.626 x 10 -34 J s)(2.998 x 108 m/s) hc = = 5.7578 x 10-19 J -7 3.45 x 10 m Now, subtract the work function of Ca = 4.34 x 10-19 J (Problem 7.69) from Ep: 5.7578 x 10-19 J - 4.34 x 10-19 J = 1.4178 x 10-19 J Note that, for this situation, E = 1/2mv2. Recall that the mass of the electron is 9.1095 x 10-31 kg. Now calculate speed, v: v = 7.72 2E = m 2 x 1.4178 x 10 -19 J 9.1095 x 10 -31 kg = 5.579 x 105 = 5.58 x 105 m/s First calculate Ep, the energy of the 285-nm photon, noting that it is equivalent to 2.85 x 10-7 m. Ep = (6.626 x 10 -34 J s)(2.998 x 108 m/s) hc = = 6.970 x 10-19 J -7 2.85 x 10 m Now, subtract the work function of Mg = 5.90 x 10-19 J (Problem 7.70) from Ep: 6.970 x 10-19 J - 5.90 x 10-19 J = 1.070 x 10-19 J Note that, for this situation, E = 1/2mv2. Recall that the mass of the electron is 9.1095 x 10-31 kg. Now calculate speed, v: v = 2E = m 2 x 1.070 x 10 -19 J 9.1095 x 10 -31 kg = 4.847 x 105 = 4.85 x 105 m/s 256 CHAPTER 7 7.73 This is a transition from the n = 5 level to the n = 2 level. Solve the Balmer equation using Bohr's approach. E5 = - RH 5 2 = - RH ; 25 E2 = - RH 2 2 = - RH 4 21RH - R - R h = H - H = 100 25 4 = = 7.74 21R H 100 h = 2.179 x 10 -18 J 21 x = 6.9059 x 1014 /s -34 100 6.626 x 10 J s c 2.998 x 108 m/s = = 4.341 x 10-7 = 4.34 x 10-7 m (434 nm) 14 6.9059 x 10 /s This is a transition from the n = 3 level to the n = 2 level. Solve the Balmer equation using Bohr's approach. E3 = - RH 32 = - RH ; 9 E2 = - RH - RH = 4 22 5 RH - R - R h = H - H = 36 9 4 = = 7.75 5 RH 36 h = 2.179 x 10 -18 J 5 x = 4.5674 x 1014 /s 36 6.626 x 10 -34 J s c 2.998 x 108 m/s = = 6.563 x 10-7 = 6.56 x 10-7 m (656 nm) 14 4.5674 x 10 /s Use 397 nm = 3.97 x 10-7 m, and convert to frequency and then to energy. = c 2.998 x 108 m/s = = 7.551 x 1014 /s 3.97 x 10 -7 m E = h = (6.626 x 10-34 Js) x (7.551 x 1014) = 5.0037 x 10-19 J (continued) QUANTUM THEORY OF THE ATOM 257 Substitute this energy into the Balmer formula recalling that the Balmer series is an emission spectrum, so E is negative: E2 = - RH 2 2 = - RH ; 4 - RH - RH - 2 = - RH 4 n i - RH Ei = ni 2 1 1 = E (of line) 4 n2 i 1 -19 J - 1 = 5.0037 x 10 = 0.22963 2 18 4 n 2.179 x 10 J i 1 n i2 = 1 - 0.22963 = 0.02036 4 1 ni = 0.02036 7.76 1/2 = 7.007 = 7.0 (= n) Convert 9.50 x 10-8 m into frequency and then to energy. = c 2.998 x 108 m/s = = 3.1557 x 1015 /s -8 9.50 x 10 m E = h = (6.626 x 10-34 Js) x (3.1557 x 1015) = 2.0910 x 10-18 J Substitute this energy into the Balmer formula recalling that the Lyman series is an emission spectrum, so E is negative, and nf = 1: E1 = - RH 2 1 = - RH ; 1 - RH - RH - 2 = - RH 1 n i Ei = - RH ni 2 1 1 = E (of line) 1 n 2 i -18 J 1 - 1 = 2.0910 x 10 = 0.95962 2 -18 n 2.179 x 10 J i (continued) 258 CHAPTER 7 1 n i2 = 1 - 0.95962 = 0.04037 1 ni = 0.04037 7.77 12 = 4.976 = 5 (= n) Employ the Balmer formula using Z = 2 for the He+ ion. E 3 = ( 2 )2 - RH 3 2 = (4) - RH ; 9 E 2 = ( 2 )2 - RH - RH = (4) 2 4 2 -R -R 5RH 4 H - 4 H = 4 x = h 36 4 9 The frequency of the radiation is = = 7.78 4 x 5 RH 36 h = 2.179 x 10 -18 J 20 x = 1.8269 x 1015 /s 36 6.626 x 10 -34 J s c 2.998 x 108 m/s = = 1.6409 x 10-7 = 1.64 x 10-7 m (164 nm; near UV) 15 1.8269 x 10 /s Employ the Balmer formula using Z = 3 for the Li2+ ion. E 4 = ( 3 )2 - RH 4 2 = (9) - RH ; 16 E 3 = ( 3 )2 - RH 3 2 = (9) - RH 9 -R -R 7RH 9 H - 9 H = 9 x = h 16 9 144 = = 9 x 7 RH 144 h = 2.179 x 10 -18 J 63 x = 1.4387 x 1015 /s 144 6.626 x 10 -34 J s c 2.998 x 108 m/s = = 2.0837 x 10-7 = 2.08 x 10-7 m (208 nm; UV) 1.4387 x 1015 /s QUANTUM THEORY OF THE ATOM 259 7.79 First, use the wavelength of 10.0 pm (1.00 x 10-11 m) and the mass of 9.1095 x 10-31 kg to calculate the velocity, v. Then, use the kinetic energy equation to calculate kinetic energy from velocity. v = 6.626 x 10 -34 kg m 2 / s h = = 7.2737 x 107 m/s -31 -11 m 9.1095 x 10 kg x 1.00 x 10 m E = 1/2mv2 = 1/2 x (9.1095 x 10-31 kg) x (7.273 x 107 m/s)2 = 2.409 x 10-15 J EeV = 2.409 x 10-15 J x 7.80 1 eV 1.602 x 10 -19 J = 1.503 x 104 = 1.50 x 104 eV First, use the wavelength of 10.0 pm (1.00 x 10-11 m) and the mass of 1.675 x 10-27 kg to calculate the velocity, v. Then, use the kinetic energy equation to calculate kinetic energy from velocity. v = 6.626 x 10 -34 kg m 2 / s h = = 3.9558 x 104 m/s -27 -11 m 1.675 x 10 kg x 1.00 x 10 m E = 1/2mv2 = 1/2 x (1.675 x 10-27 kg) x (3.9558 x 104 m/s)2 = 1.3105 x 10-18 J EeV = 1.3105 x 10-18 J x 1 eV 1.602 x 10 -19 J = 8.1803 = 8.18 eV 7.81 a. Five b. Seven c. Three d. One 7.82 a. Seven b. Nine c. One d. Three 7.83 The possible subshells for the n = 6 shell are 6s, 6p, 6d, 6f, 6g, and 6h. 7.84 The possible subshells for the n = 7 shell are 7s, 7p, 7d, 7f, 7g, 7h, and 7i. 260 CHAPTER 7 Solutions to Cumulative-Skills Problems 7.85 First, use Avogadro's number to calculate the energy for one Cl2 molecule. 1000 J 239 kJ 1 mol x x = 3.9687 x 10-19 J/molecule 23 1 kJ 1 mol 6.022 x 10 molecules Then, convert energy to frequency and finally to wavelength. 7.86 = E 3.9687 x 10 -19 J = = 5.9897 x 1014 /s 34 h 6.626 x 10 Js = c 2.998 x 108 m/s = = 5.0052 x 10-7 m (501 nm; visible region) 5.9897 x 1014 /s First, use Avogadro's number to calculate the energy per H2 molecule. 1000 J 432 kJ 1 mol x x = 7.1736 x 10-19 J/molecule 1 kJ 1 mol 6.022 x 1023 molecules Then, convert energy to frequency and finally to wavelength. 7.87 = E 7.1736 x 10 -19 J = = 1.0826 x 1015 /s -34 h 6.626 x 10 J s = c 2.998 x 108 m/s = = 2.7691 x 10-7 m (277 nm; UV) 15 1.0826 x 10 /s First, calculate the energy needed to heat the 0.250 L of water from 20.0°C to 100.0°C. 0.250 L x 1000 g 4.184 J x x (100.0 C - 20.0C) = 8.368 x 104 J 1L (g C) Then, calculate the frequency, the energy of one photon, and the number of photons. = 2.998 x 108 m/s c = = 2.398 x 109 /s 0.125 m (continued) QUANTUM THEORY OF THE ATOM 261 E of one photon = h = (6.626 x 10-34 Js) x (2.398 x 109/s) = 1.589 x 10-24 J 1 photon No. photons = h = 8.368 x 104 J x 1.589 x 10 -24 = 5.2656 x 1028 J = 5.27 x 1028 photons 7.88 First, calculate the energy needed to heat the 1.00 L of water from 20.0°C to 30.0°C. 1.00 L x 1000 g 4.184 J x x (30.0 C - 20.0C) = 4.184 x 104 J 1L (g C) Then, calculate the frequency, the energy of one photon, and the number of photons. = c 2.998 x 108 m/s = = 1.0707 x 1014 /s -6 2.80 x 10 m E of one photon = h = (6.626 x 10-34 Js) x (1.0707 x 1014/s) = 7.0945 x 10-20 J 1 photon No. photons = h = 4.184 x 104 J x -20 7.0945 x 10 = 5.8974 x 1023 J 23 = 5.90 x 10 photons 7.89 First, write the following equality for the energy to remove one electron, E removal: Eremoval = E425 nm - Ek of ejected photon Use E = h to calculate the energy of the photon. Then, recall that Ek, the kinetic energy, is 1/2mv2. Use this to calculate Ek. E425 nm = (6.626 x 10 -34 J s)(2.998 x 108 m/s) hc = = 4.6740 x 10-19 J 4.25 x 10 -7 m Ek = 1/2mv2 = 1/2 x (9.1095 x 10-31 kg) x (4.88 x 105 m/s)2 = 1.0846 x 10-19 J Subtract to find Eremoval, and convert it to kJ/mol: Eremoval = 4.6740 x 10-19 J - (1.0847 x 10-19 J) = 3.5893 x 10-19 = 3.59 x 10-19 J/electron (continued) 262 CHAPTER 7 Eremoval = 3.5893 x 10-19 J 1 e- x 6.022 x 1023 e1 kJ x = 2.161 x 102 1 mol 1000 J = 2.16 x 102 kJ/mol 7.90 First, write the following equality for the energy to remove one electron, Eremoval: Eremoval = E405 nm - Ek of ejected electron Use E = h to calculate the energy of the photon. Then, recall that Ek, the kinetic energy, is 1/2mv2. Use this to calculate Ek. E405 nm = (6.626 x 10 -34 J s)(2.998 x 108 m/s) hc = = 4.9048 x 10-19 J -7 4.05 x 10 m Ek = 1/2mv2 = 1/2 x (9.1095 x 10-31 kg) x (3.36 x 105 m/s)2 = 5.142 x 10-20 J Subtract to find Eremoval, and convert it to kJ/mol: Eremoval = 4.9048 x 10-19 - 5.142 x 10-20 = 4.3906 x 10-19 = 4.39 x 10-19 J/electron Eremoval = 4.3906 x 10-19 J 1 e- x 6.022 x 1023 e1 kJ x = 264.4 1 mol 1000 J = 264 kJ/mol 7.91 First, calculate the energy, E, in joules using the product of voltage and charge: E = (4.00 x 103 V) x (1.602 x 10-19 C) = 6.408 x 10-16 J Now, use the kinetic energy equation, Ek = 1/2mv2, and solve for velocity: v = = 2 Ek = m 2 x 6.408 x 10 -16 J 9.1095 x 10 -31 = 3.7508 x 107 m/s kg h 6.626 x 10 -34 J s = = 1.939 x 10-11 -31 7 mv (9.1095 x 10 kg) x (3.7508 x 10 m/s) = 1.94 x 10-11 m (19.4 pm) QUANTUM THEORY OF THE ATOM 263 7.92 First, calculate the energy, E, in joules using the product of voltage and charge: E = (1.00 x 104 V) x (1.602 x 10-19 C) = 1.602 x 10-15 J Now, use the kinetic energy equation Ek = 1/2mv2, and solve for velocity: v = = 2 Ek = m 2 x 1.602 x 10 -15 J 9.1095 x 10 -31 = 5.9306 x 107 m/s kg h 6.626 x 10 -34 J s = = 1.226 x 10-11 -31 7 mv (9.1095 x 10 kg) x (5.9306 x 10 m/s) 1.23 x 10-11 m (12.3 pm)