Fri | Sep 7, 2007 Chapter 4: Types of Chemical Reactions
advertisement
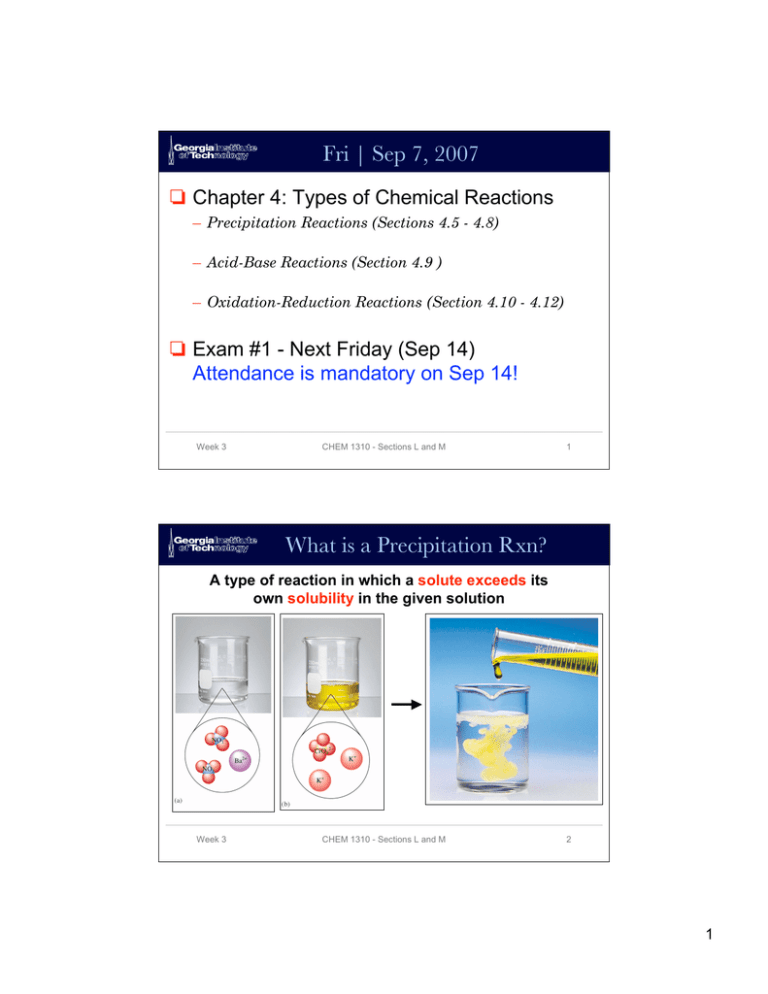
Fri | Sep 7, 2007 Chapter 4: Types of Chemical Reactions – Precipitation Reactions (Sections 4.5 - 4.8) – Acid-Base Reactions (Section 4.9 ) – Oxidation-Reduction Reactions (Section 4.10 - 4.12) Exam #1 - Next Friday (Sep 14) Attendance is mandatory on Sep 14! Week 3 CHEM 1310 - Sections L and M 1 What is a Precipitation Rxn? A type of reaction in which a solute exceeds its own solubility in the given solution Week 3 CHEM 1310 - Sections L and M 2 1 Precipitation Conditions Under what types of conditions will precipitation occur? Addition of Excess Solute Removal of Solvent Change of Solvent Change of Temperature Week 3 CHEM 1310 - Sections L and M 3 Questions re: Precipitation Rxns How could one predict what product would precipitate in a given precipitation reaction? How would one write a chemical equation to represent a precipitation reaction Week 3 CHEM 1310 - Sections L and M 4 2 Predicting ID of Precipitant Know the chemical formulas of each reactant “Swap” ions Know the solubility rules Potassium Chromate (yellow) Silver Nitrate (clear solution) What is the red precipitate? Week 3 CHEM 1310 - Sections L and M 5 Predicting ID of Precipitant Know the chemical formulas of each reactant “Swap” ions Know the solubility rules K2CrO4 AgNO3 2 K+ and 1 Ag+ and 1 CrO421 NO3- Products: Ag2CrO4 and KNO3 What is the red precipitate? Week 3 CHEM 1310 - Sections L and M 6 3 Solubility Rules Table 4.1 from Text - VERY IMPORTANT! Week 3 CHEM 1310 - Sections L and M 7 Predicting ID of Precipitant Know the chemical formulas of each reactant “Swap” ions Know the solubility rules K2CrO4 AgNO3 2 K+ and 1 Ag+ and 1 CrO421 NO3- Products: Ag2CrO4 and KNO3 What is the red precipitate? Ag2CrO4 Week 3 CHEM 1310 - Sections L and M 8 4 Chemical Equations How would one write a balanced chemical equation describing the precipitation reaction shown? K2CrO4 + AgNO3 Ions Reacting 2 mol K+ 1 mol CrO421 mol Ag+ 1 mol NO3- Ag2CrO4 + KNO3 Ions in Products 1 mol K+ 1 mol CrO422 mol Ag+ 1 mol NO3- Balance ions by visual inspection… Week 3 CHEM 1310 - Sections L and M 9 Chemical Equations How would one write a balanced chemical equation describing the precipitation reaction shown? K2CrO4 + 2AgNO3 Ag2CrO4 + 2KNO3 Ions Reacting 2 mol K+ 1 mol CrO422 mol Ag+ 2 mol NO3- Ions in Products 2 mol K+ 1 mol CrO422 mol Ag+ 2 mol NO3- Balanced Week 3 CHEM 1310 - Sections L and M 10 5 Types of Acids and Bases Arrhenius Acids and Bases • Acids are H+ donors • Bases are OH- donors Arrhenius Broadened Definition • • Week 3 Putting brackets around a term means “concentration of” the term Acids increase [H+] Bases increase [OH-] CHEM 1310 - Sections L and M 11 Acid-Base Titrations What is a titration? Systematic addition of a reagent until all of another reagent is consumed. Detection is visual Indicator = Phenolphthalein Acidic pH - Colorless Neutral pH - Pink Week 3 CHEM 1310 - Sections L and M Base Acid 12 6 Acid-Base Titrations What is [NaOH] when 37.65 mL of NaOH are used to titrate 0.6135g of KHC8H4O4? Known Information • # mol NaOH = # mol KHC8H4O4 • [NaOH] = # mol NaOH / vol soln • Vol Soln = 37.65 x 10-3L 0.6135 g X [NaOH] = Week 3 1 mol KHC8H4O4 204.22g KHC8H4O4 3.004 x 10-3 mol 37.65 x 10-3 L = 3.004 x 10-3 mol = 0.07979 M NaOH CHEM 1310 - Sections L and M 13 Acid & Base Titrations EXAMPLE 4-10 from Text What volume of 0.100 M HCl is needed to neutralize 25.0 mL of a 0.350 M NaOH solution? • Write a balanced chemical equation from the word problem • Use rules of stoichiometry to relate terms • Solve via calculation Week 3 CHEM 1310 - Sections L and M 14 7 Redox Reactions Redox: a class of reactions characterized by the transfer of electrons. 0 0 +2 -2 2 Mg(s) + O2(g) → 2 MgO(s) ↓ loss ↑ gain 2 x 2e- = 1 x 2 x 2e- Magnesium is oxidized: it gives up electrons as the charge on its atoms increases from 0 to +2. Oxygen is reduced: it gains electrons as the charge on its atoms decreases from 0 to -2 (i.e., becomes more negative). Week 3 CHEM 1310 - Sections L and M 15 Oxidation Number STUDY Table 4.3 Week 3 CHEM 1310 - Sections L and M 16 8 Oxidation Number Oxidation Numbers (also called oxidation states) are determined for the atoms in covalently bonded compounds by applying the following set of 3 simple rules: 1. The oxidation number of the atoms in a neutral molecule must all up to 0; those in an ion must add up to the charge on the ion. Week 3 CHEM 1310 - Sections L and M 17 Oxidation Number Oxidation Numbers (also called oxidation states) are determined for the atoms in covalently bonded compounds by applying the following set of simple rules: 2. Alkali metal (Group I) atoms have oxidation number +1, alkaline earth (Group II) atoms have oxidation number +2 in their compounds; atoms of Group III elements usually have oxidation number +3 in their compounds. Week 3 CHEM 1310 - Sections L and M 18 9 Oxidation Number Oxidation Numbers (also called oxidation states) are determined for the atoms in covalently bonded compounds by applying the following set of simple rules: 3. Fluorine always has an oxidation number of -1 in its compounds. The other halogens have oxidation number -1 in their compounds, except in compounds with oxygen and with other halogens, in which they can have positive oxidation numbers. Week 3 CHEM 1310 - Sections L and M 19 Nomenclature for Redox Rxns Oxidation Number Change Electron Change Oxidation Increase Loss of Electrons Reduction Decrease Gain of Electrons Oxidizing Agent, does the oxidizing Decrease Picks Up electrons Reducing Agent, does the reducing Increase Supplies Electrons Substance Oxidized Increase Loses Electrons Substance Reduced Decrease Gains Electrons Term Week 3 CHEM 1310 - Sections L and M 20 10 Summary Know meanings of terms Know how to predict a precipitate Be able to calculate terms related to acid-base titrations Determine oxidation state of an atom Write and balance chemical equations for precipitation, acid-base rxns and redox rxns Week 3 CHEM 1310 - Sections L and M 21 Study Tips Read text Work problems from text Take practice exams online Review lecture notes Exam #1 Friday, Sep 14 Need #2 Pencil Calculator Crib Sheet Study in groups Week 3 CHEM 1310 - Sections L and M 22 11