Crystal Structures of Interest • Elemental solids: • Binary compounds
advertisement
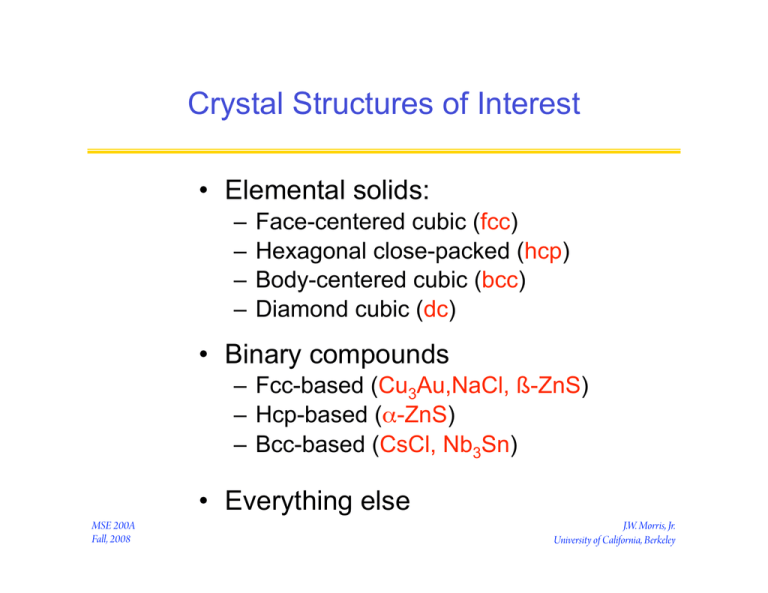
Crystal Structures of Interest
• Elemental solids:
– Face-centered cubic (fcc)
– Hexagonal close-packed (hcp)
– Body-centered cubic (bcc)
– Diamond cubic (dc)
• Binary compounds
– Fcc-based (Cu3Au,NaCl, ß-ZnS)
– Hcp-based (α-ZnS)
– Bcc-based (CsCl, Nb3Sn)
• Everything else
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
fcc and hcp from Stacking
Close-Packed Planes
A
A
B
A
A
A
B
B
C
A
A
A
B
C
A
A
BB
C
A
A
C
C
→
C
A
B
A
AB
A
A
A
B
→
C
C
A
A
B
A
B
C
A
A
ABA = hcp
A
B
C
C
A
A
A
B
B
C
A
A
A
• There are two ways to stack spheres
• The sequence ABA creates hcp
• The sequence ABC creates fcc MSE 200A
Fall, 2008
The image
cannot be
displayed. Your
computer may
not have
enough
memory to
open the
image, or the
image may
ABC = fcc
J.W. Morris, Jr.
University of California, Berkeley
Hexagonal Close-Packed
MSE 200A
Fall, 2008
•
HCP does not have a primitive cell
•
Common in
•
Anisotropy limits engineering use of these elements
– 2 atoms in primitive cell of hexagonal lattice
– 6 atoms in cell drawn to show hexagonal symmetry
– Divalent elements: Be, Mg, Zn, Cd
– Transition metals and rare earths: Ti, Zr, Co, Gd, Hf, Rh, Os
J.W. Morris, Jr.
University of California, Berkeley
Face-Centered Cubic Structure
ABC stacking
Fcc cell
View along diagonal (<111>)
•
FCC is cubic stacking of close-packed planes ({111})
•
Common in
– 1 atom in primitive cell; 4 in cell with cubic symmetry
– <110> is close-packed direction
– Natural and noble metals: Cu, Ag, Au, Pt, Al, Pb
– Transition metals: Ni, Co, Pd, Ir
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
Interstitial Sites:
Octahedral Voids in fcc
MSE 200A
Fall, 2008
•
Octahedral interstitial site is equidistant from six atoms
•
There are 4 octahedral voids per fcc cell
– “Octahedral void”
– Located at {1/2,1/2,1/2} and {1/2,0,0}
– One per atom
J.W. Morris, Jr.
University of California, Berkeley
Interstitial Sites:
Tetrahedral Voids in FCC
• Tetrahedral site is equidistant from four atoms
– “tetrahedral void”
– Located at {1/4,1/4,1/4} - center of cell octet
• There are 8 tetrahedral voids per fcc cell
– Two per atom
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
Interstitial Sites:
Voids between Close-packed Planes
A
B
C
C
A
A
A
B
B
C
A
A
A
A
B
C
C
A
A
A
B
B
C
A
A
•
In both FCC and HCP packing:
•
Stacking including voids:
A
– Tetrahedral void above and below each atom (2 per atom)
– Octahedral void in third site between planes
– Fcc: ...(aAa)c(bBb)a(cCc)b(aAa)…
– Hcp: ...(aAa)c(bBb)c(aAa)… (octahedral voids all on c-sites)
⇒ Size and shape of voids are the same in fcc and hcp
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
The Diamond Cubic Structure
•
Fcc plus atoms in 1/2 of tetrahedral voids
•
DC is the structure of the Group IV elements
– Close-packed plane stacking is ...AaBbCc… or ... aAbBcC...
- Each atom has four neighbors in tetrahedral coordination
- Natural configuration for covalent bonding
– C, Si, Ge, Sn (grey)
– Are all semiconductors or insulators
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
Binary Compounds: Examples
• Substitutional:
– Bcc: CsCl
– Fcc: Cu3Au
• Interstitial:
–
–
–
–
MSE 200A
Fall, 2008
Fcc octahedral: NaCl
Fcc tetrahedral: ß-ZnS
Hcp tetrahedral: α-ZnS
Bcc tetrahedral: Nb3Sn (A15)
J.W. Morris, Jr.
University of California, Berkeley
FCC Substitutional: Cu3Au
• FCC parent
– Stoichiometric formula A3B
– B-atoms on edges
– A-atoms on faces
• Found in:
– Intermetallic compounds (Cu3Au)
– As “sublattice” in complex ionics
• E.g., “perovskites”
– BaTiO3 (ferroelectric)
– YBa2Cu3O7 (superconductor)
• Lattices of + and - ions must
interpenetrate since like ions cannot
be neighbors
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
FCC Octahedral Interstitial: NaCl
• FCC parent
–
–
–
–
Stoichiometric formula AB
A-atoms on fcc sites
B-atoms in octahedral voids
Either can be “A”
• Found in:
– Ionic compounds:
• NaCl, MgO (RB/RA ~ 0.5)
• “Perovskites” (substitutional
ordering on both sites)
– Metallic compounds
• Carbonitrides (TiC, TiN, HfC)
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
FCC Tetrahedral Interstitial: ß-ZnS
• Binary analogue of DC
– Stoichiometric formula AB
– A-atoms on fcc sites
– B-atoms in 1/2 of tetrahedral voids
• AaBbCc
– Either element can be “A”
• Found in:
– Covalent compounds:
• GaAs, InSb, ß-ZnS, BN
– Ionic compounds:
• AgCl
• Large size difference (RB/RA < .414)
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
Hcp Tetrahedral Interstitial: α-ZnS
• Hexagonal analogue of ß-ZnS
– Stoichiometric formula AB
– A-atoms on hcp sites
– B-atoms in 1/2 of tetrahedral voids
• AaBbAaBb
– Either element can be “A”
• Found in:
– Covalent compounds:
• ZnO, CdS, α-ZnS, “Lonsdalite” C
– Ionic compounds:
• Silver halides
• Large size difference (RB/RA < .414)
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
Description of
Complex Crystal Structures
• Most crystals can be referred to a close-packed frame
– Fcc or hcp network
– Possibly plus small distortions along symmetry axes
• Cubic → tetragonal (edge unique),
• Cubic → rhombohedral (diagonal unique)
• Atoms in ordered configurations in
– Substitutional sites
– Interstital sites: octahedral or tetrahedral
– Vacancies are useful as “atoms” to complete the configuration
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
Hierarchical Description of
Complex Crystal Structures
•
Construct planar layers
•
Identify ordered pattern
•
Order layers
– Network (fcc or hcp)
– Interstitial planes that contain atoms
– Primary and interstitial planes
– Pattern is the same on all planes
– Including vacancies, if necessary, as species
– Physical pattern (fcc or hcp)
– Chemical pattern
• composition may change from layer to layer (differentiation)
– Stacking pattern is the same for network and interstitial layers
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
Representation of Crystal Structures
• The basic pattern is L t o t L at 0, 1/4, 1/2, 3/4, 1)
FCC:
HCP:
AbcaBcabCabcA
AbcaBacbAbcaB
• Levels of representation
– 1 - planar order: X, XY. X2Y, X3Y
– 2 - interstitial character: octahedral, tetrahedral(1,2)
– 3 - stacking pattern: fcc, hcp, polytypic, hexagonal
– 4 - differentiation: undifferentiated, differentiated
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
Substitutional X-Compounds
• Undifferentiated
– All atoms are the same: fcc, hcp, polytypes (e.g., ABCBABCBA…)
• Differentiated
– Planes of atoms alternate: CuPt, WC
– Note that cubic symmetry is broken in CuPt: rhombohedral
^
^
MSE 200A
Fall, 2008
^
^
^
^
= Cu
=W
= Pt
=C
J.W. Morris, Jr.
University of California, Berkeley
Octahedral Interstital X-Compounds
•
Undifferentiated
•
Differentiated
= Na
= As
= Cl
= Ni
– Fcc or hcp planes alternate with filled octahedral planes: NaCl, NiAs
– Note that o-sites in NiAs are ccc, can tell which atom is in octahedral hole
– Alternate lattice or interstitial planes differ
– CdI2: like NiAs but octahedral Cd planes alternate with vacant planes
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
Tetrahedral(I) X-compounds
= Zn
= Zn
=S
=S
• Lattice planes plus one plane of tetrahedral voids
• Examples:
– Unary: diamond cubic, hexagonal diamond (Lonsdaleite)
– Binary: α-ZnS, β-ZnS
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
Tetrahedral(II) X-Compounds
= Ca
=F
• Lattice planes plus planes on both tetrahedral sites
• Fcc-based: CaF2 (flourite) and Li2O
• Hcp-based: none known
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
Distributions of XY in a plane
• XY:
– 3 basic patterns
– Label I, II, III
• X2Y:
– 1 basic pattern
• X3Y:
– 2 basic patterns
– Label I, II
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
Binary (XY) Patterns in a Plane
XY(I)
(common)
Note: all planes in the
stacking have the
same type of order.
XY(II)
XY(III)
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
Substitutional XY undifferentiated
= Cu
= Au
• Only known example is fcc-based CuAu(I)
– Has the XY(I) pattern in every plane
– Creates structure in which Cu, Au fill alternate (100) planes
– Cubic symmetry lost: tetragonal
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
Differentiated Substitutional XY
C
A
B
= Cu
= Pt
• Fcc example: CuPt3
– CuPt planes in XY(I) order alternate with Pt planes
• Note Pt plane has XY(I) pattern with X=Y
– Stacking: A(CuPt)B(Pt)C(CuPt)A(Pt)B(CuPt)C(Pt)
– Cubic symmetry broken: rhombohedral
• No hcp-based examples
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
FCC-based Octahedral XY
= Fe
=N
• (Fe,Ni)2N
– Fcc solution of Fe and Ni
– XY(I) pattern of N and ∅ on octahedral layers
– Cubic symmetry broken: tetragonal
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
HCP-based Octahedral XY
• Many MO2 oxides are hcp
–
–
–
–
TiO 2
å - PbO 2
= O at A-sites 0,1
= O at B-sites (1/2)
= M at c1 sites (1/4)
= M at c2 sites (3/4)
FeO(OH)
MSE 200A
Fall, 2008
O on hcp sites
M∅ on octahedral planes
M alternates to fill all sites
Pattern Ac1Bc2A
• Examples:
– TiO2 (rutile) = Ti in XY(I), O
on HCP sites
– α-PbO2 = Pb in XY(II)
– FeO(OH) (geothite) = Fe in
XY(III), O and (OH) planes
alternate
J.W. Morris, Jr.
University of California, Berkeley
X2Y Pattern
• Note that 2d unit cell contains three atoms
– Cell outlined in red
• Requires three planes for symmetric coverage of sites
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
Octahedral X2Y: Corundum
• Analogue of NiAs
– X2Y order in the plane
• Examples:
– Al2O3
– FeTiO3
MSE 200A
Fall, 2008
…c1Ac2Bc3Ac1B…
Note 6-layer repeat pattern
J.W. Morris, Jr.
University of California, Berkeley
Most Common X3Y Pattern
• Note that 2d unit cell contains four atoms
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
Octahedral X3Y Perovskite
• Perovskite: CaTiO3
• Also Fe4N, Fe8N, Ni4N
• Also YBCO and other oxide superconductors
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley
Defects in Crystals
• Imperfections are present in all real crystals
• Often, they are added to control properties
– Materials engineering is largely “defect”
engineering
• Classify defects by dimension
– Point defects: solute atoms (strength, conductivity)
– Line defects: dislocations (plastic deformation)
– Surface defects: external surface (crystal shape)
– Volume defects: voids, inclusions (fracture)
MSE 200A
Fall, 2008
J.W. Morris, Jr.
University of California, Berkeley