Study Guide for Quiz 2
advertisement
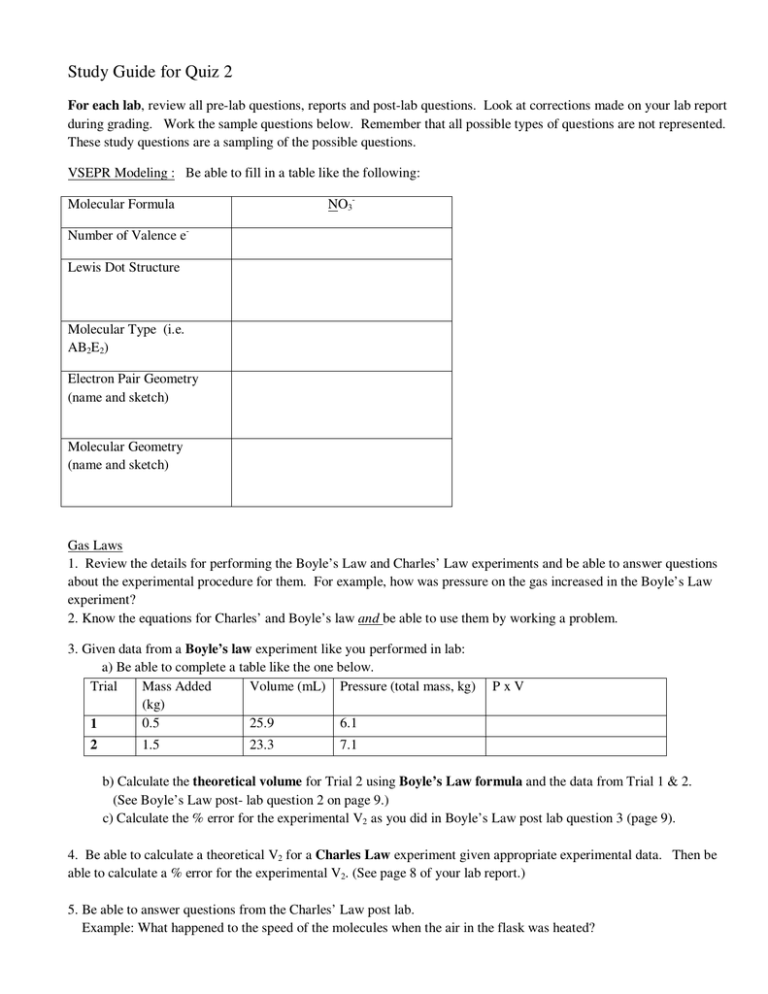
Study Guide for Quiz 2 For each lab, review all pre-lab questions, reports and post-lab questions. Look at corrections made on your lab report during grading. Work the sample questions below. Remember that all possible types of questions are not represented. These study questions are a sampling of the possible questions. VSEPR Modeling : Be able to fill in a table like the following: NO3- Molecular Formula Number of Valence eLewis Dot Structure Molecular Type (i.e. AB2E2) Electron Pair Geometry (name and sketch) Molecular Geometry (name and sketch) Gas Laws 1. Review the details for performing the Boyle’s Law and Charles’ Law experiments and be able to answer questions about the experimental procedure for them. For example, how was pressure on the gas increased in the Boyle’s Law experiment? 2. Know the equations for Charles’ and Boyle’s law and be able to use them by working a problem. 3. Given data from a Boyle’s law experiment like you performed in lab: a) Be able to complete a table like the one below. Trial Mass Added Volume (mL) Pressure (total mass, kg) (kg) 0.5 25.9 6.1 1 2 1.5 23.3 PxV 7.1 b) Calculate the theoretical volume for Trial 2 using Boyle’s Law formula and the data from Trial 1 & 2. (See Boyle’s Law post- lab question 2 on page 9.) c) Calculate the % error for the experimental V2 as you did in Boyle’s Law post lab question 3 (page 9). 4. Be able to calculate a theoretical V2 for a Charles Law experiment given appropriate experimental data. Then be able to calculate a % error for the experimental V2. (See page 8 of your lab report.) 5. Be able to answer questions from the Charles’ Law post lab. Example: What happened to the speed of the molecules when the air in the flask was heated? Chemistry of Copper Lab In general: Be able to identify the type of reaction, predict products, write correct formulas, and balance equations. Know the colors of all the copper containing products. Review the experiment and your results. Make sure you understand each step that you performed. For example, why was reaction 1 performed in the hood? 1. Classify the type of reaction and balance the equation: Cu + O2 → CuO 2. Classify the reaction, predict the products, balance the equation and write the net ionic equation for Cu (s) + AgNO3 (aq) → 3. Calculate the percent yield if you started with 1.56 g of copper wire and recovered 0.88 g of copper. 4. What was the color of CuO? Representing Organic Structures Be able to write expanded structures from line structures and vice versa. (p. 11 in lab) 1. Are the following compounds isomers, the same compound or neither? (Two compounds are isomers if they have the same molecular formula, but different structure.) H C H H H C H H C C H H H H C C H C H H H H H H C H H H 2. Draw a flat picture of a six carbon alkane with the longest possible chain of carbon atoms. Indicate the name and formula. Then rearrange the carbon chain to form an isomer. isomer longest chain # of C in main chain 2 3 4 5 6 7 name: formula: ________________ 3. Draw a perspective line drawing for cis-1,2-dichlorocyclopentane (question 1, postlab). 4. Draw flat structures for two isomers of dibromopropane (C3H6Br2) and name them. 5. What is necessary for a carbon to be chiral? 6. Draw a perspective view of an alkene with the formula C2H2Cl2 Some Answers VSEPR Molecular Formula Number of Valence e- NO324 valence electron _ Lewis Dot Structure O O N O name ethane propane butane pentane hexane heptane Molecular Type (i.e. AB2E2) Electron Pair Geometry (name and sketch) Molecular Geometry (name and sketch) AB3 trigonal planar trigonal planar Gas Laws 1. The pressure on the gas was increased by adding weights on top of the syringe. 2. Charles’ Law, V1 / T1 = V2 / T2 Boyle’s law, P1V1 = P2V2 3. Given data from a Boyle’s law experiment like you performed in lab: a) Be able to complete a table like the one below. Trial Mass Added Volume (mL) Pressure (total mass, kg) (kg) 0.5 25.9 6.1 1 2 PxV (157.99) 160 kg . mL 1.5 23.3 7.1 (165.43) 170 kg . mL b) Calculate the theoretical volume for Trial 2 using Boyle’s Law formula and the data from Trial 1 & 2. First list the values for each pressure and volume: P1 = 6.1 kg, V1 = 25.9 mL, and P2 = 7.1 kg Boyle’s Law: P1V1 = P2V2 V2 = P1 V1 P2 = 6.125.9 7.1 = (22.25211) 22 mL 5. The speed of the molecules increased when the air in the flask was heated. Chemistry of Copper Lab 1. Classify the type of reaction and balance the equation: 2 Cu + O2 → 2 CuO Combination or synthesis 2. Classify the reaction, predict the products, balance the equation and write the net ionic equation for Single replacement Cu (s) + 2 AgNO3 (aq) → 2 Ag + Cu(NO3)2 (This reaction proceeds because copper is more active than silver.) NIE Cu (s) + 2 Ag+ (aq) → 2 Ag + Cu2+ 3. Calculate the percent yield if you started with 1.56 g of copper wire and recovered 0.88 g of copper. . . x 100% = 56.4% rounds to 56% Representing Organic Structures 1. Are the following compounds isomers, the same compound or neither? (Two compounds are isomers if they have the same molecular formula, but different structure.) H H C C H H C C H H C H H H H C H C H H H H H C H H H H These are isomers. They have the same number of each type of atom. But they are connected in different ways to give different structures. 2. Draw a flat picture of a six carbon alkane with the longest possible chain of carbon atoms. Indicate the name and formula. Then rearrange the carbon chain to form an isomer. H H H H H H C C C C C C H H H H H H H H H H H H H C C C C C H H H C H H H H H H Isomer Longest chain name: hexane formula: C6H14 3. Draw a perspective line drawing and Haworth projection for cis-1,2-dichlorocyclopentane (question 1, postlab). Cl Cl 4. Draw flat structures for two isomers of dibromopropane (C3H6Br2) and name them. Br H Br H C C C H H H 1,2-dibromopropane H Br Br H H C C C H H H 1,1-dibromopropane H (How many more isomers are there?)



