Making Gluep

Making Gluep
1
Thomas M. Moffett Jr., SUNY Plattsburgh, 2010
Polymers are large molecules with structures that resemble chains. They are formed from a smaller molecule (monomer) repeating many times, like the links on a chain. All plastics are examples of polymers, in this experiment you will be creating (really modifying your own polymer).
White glue (polyvinyl acetate) consists of many repeating vinyl acetate molecules.
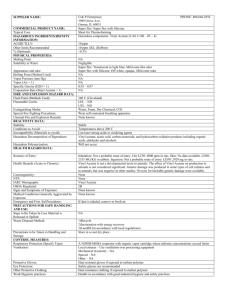
Figure 1vinyl acetate
Figure 2 A vinyl acetate trimer (three monomer units connected) on the left, and the formula showing the repeating unit in polyvinyl acetate on the right (n is a large number).
The blue atoms in the above figures show the acetate group (CH
3
COO ). Polyvinyl acetate
(PVA) molecules are very long molecules (like spaghetti). Glue is a mixture of water and PVA and water, when the water evaporates the long PVA molecules are all twisted together, this is what holds the glued item together.
In this experiment you will make gluep, in which two acetate groups are replaced by a borate ion
(B(OH)
4
). The borate ions allow for the formation of cross-links (bonds between separate polymer molecules). Cross-linking leads to a more complex structure.
1 Pronounced “Gloop”
Figure 3 Borate ion (in red) cross linking two polymer strands.
This is a fun experiment that you can do at home with younger children. The materials used in this experiment are non-toxic, and available at your local supermarket.
Material:
• Borax
• Elmer’s Glue
• Disposable cup
• Something to stir with (plastic spoons)
Procedure:
Borax Solution Prep
1.) Prepare a saturated borax solution by dissolving approximately one tablespoon of borax into one cup of water. Stir. (Some borax should remain undissolved, if not add a little more borax.)
Making Gluep
1.) Into a disposable cup add 4 teaspoons of glue and 4 teaspoons of water.
Stir the solution until it is thoroughly mixed.
2.) If you want your gluep to be colored, add one or two drops of food coloring.
3.) Add 1.5 teaspoons of borax solution to the mix, stir until it becomes a rubbery lump.
4.) Remove the gluep from the cup and knead it in your hands to remove the excess water. The gluep should no longer be sticky when it’s ready.
5.) Design and perform 5 “tests” to describe the properties of your gluep (what does it do when you stretch it slowly or quickly, etc.). You should have your tests planned before you come to lab (look online, lots of ideas).
6.) Store in a baggie
Post Lab Questions
1.) Draw a trimer of polyvinyl chloride.
2.) What is the difference between an addition polymerization reaction and a condensation polymerization reaction?
3.) Is the polymerization of vinyl acetate to form PVA an addition or condensation polymerization reaction.
Pre-lab Questions
1.) What does the borate ion do when mixed with PVA?
2.) Draw the structure of vinyl chloride ( hint: there is a chlorine instead of an acetate ).
3.) List the 5 tests that you are planning.
Formal Report
Introduction section:
• Explain what plastics and polymers are
• Show a polymerization reaction
• Effects of cross links on polymers
• Non-Newtonian fluids & Maxwell solids
Methods section – this section will be worth more than the normal 5% for this report .
• Describe the procedure for making gluep
• Describe the 5 tests that you did
Discussion/ Conclusion
• Describe the results of each test.
• Explain how the properties you described in the introduction section account for the results of your tests.
• Answers to post lab questions.
Make sure to include all references!