Alcohols, Phenols, and Ethers Introduction to alcohols Dr. Sean Bonness Chemistry Department
advertisement

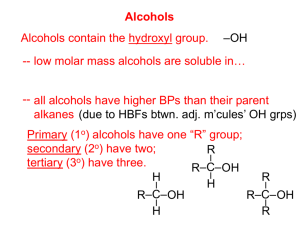
Alcohols, Phenols, and Ethers Dr. Sean Bonness Chemistry Department El Camino College Introduction to alcohols Some examples of alcohols Hydroxy (-OH) is the functional group. Alcohol has an -OH group attached to an aliphatic carbon. General formula is R-OH. Phenol has an -OH group on a benzene ring. 1 Nomenclature of alcohols and phenols IUPAC rules Step 1: Name the longest chain to which the hydroxy group is attached. Drop the final -e from the hydrocarbon and add the ending -ol. Nomenclature of alcohols and phenols IUPAC rules (cont.) Step 2: Number the longest chain to give the lower number to carbon with attached hydroxy group. Step 3: Find the position of hydroxy group by the number of the carbon atom attached to it. 2 Nomenclature of alcohols and phenols IUPAC rules (cont.) Step 4: Find and name any branches. Step 5: Combine the name and location of other groups, hydroxy group location, and long chain into final name. Nomenclature of alcohols and phenols Name the following alcohols according to IUPAC system: 3 Nomenclature of alcohols and phenols Alcohols containing two hydroxy groups are called diols. Those containing three are called triols. IUPAC rules are the same as single hydroxy alcohols, except the ending is -diol, or -triol goes on end of parent hydrocarbon. Nomenclature of alcohols and phenols Assign IUPAC names to the following alcohols 4 Nomenclature of alcohols and phenols Naming phenols Substituted phenols are usually named as derivatives of the parent compound phenol. Classification of alcohols We have seen classification of carbons on alkanes before, such as primary, secondary, and tertiary carbons. The same classification goes for the carbon that has the hydroxy group. Primary alcohols Secondary alcohols Tertiary alcohols 5 Classification of alcohols Classify the following alcohols as primary, secondary, or tertiary The –OH group is polar and capable of hydrogen bonding. This makes low molecular weight alcohols highly soluble in water. Hydrogen bonding in a water-methanol solution: Physical properties of alcohols Lower molecular weight alcohols are soluble in water. This is due to hydrogen bonding between hydroxy group and water. As molecular weight increases for alcohols, they become more “alkanelike”. Long chain alcohols are less soluble in water and more soluble in nonpolar solvents, like benzene. 6 Physical properties of alcohols Physical properties of alcohols Which member of each of the following pairs aould be more soluble in water? Why? Butane or 2-butanol 2-propanol or 2-pentanol 2-butanol or 2,3-butanediol 7 Reactions of alcohols Dehydration to produce an alkene The removal of water (dehydration) from an alcohol at 180°C is an elimination reaction that produces an alkene. Reactions of alcohols Dehydration to produce an alkene 8 Reactions of alcohols Dehydration to produce an alkene Reactions of alcohols Dehydration to produce an ether Under slightly different conditions (140°C), a dehydration reaction can occur between two alcohol molecules to produce an ether. 9 Reactions of alcohols Dehydration to produce an ether Reactions of alcohols Oxidation – the removal of hydrogen atoms 10 Reactions of alcohols Three classes of alcohols react differently to oxidizing agents. Primary alcohols Reactions of alcohols Three classes of alcohols react differently to oxidizing agents. Secondary alcohols 11 Reactions of alcohols Three classes of alcohols react differently to oxidizing agents. Tertiary alcohols 12