Elementary Particles - The Grange School Blogs
advertisement
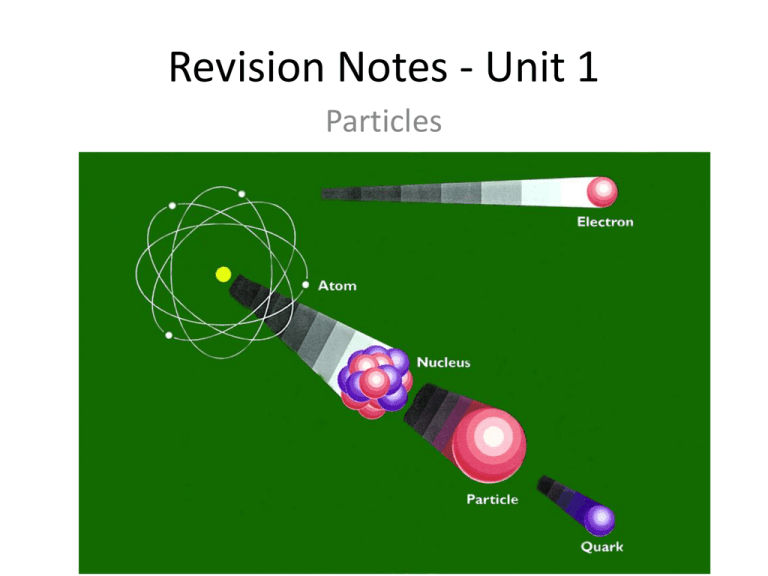
Revision Notes - Unit 1 Particles INTRODUCTION to Elementary Particle Physics * Fundamental building blocks of which all matter is composed: Elementary Particles * Pre-1930s it was thought there were just four elementary particles electron proton neutron photon 1932 positron or anti-electron discovered, followed by many other particles (muon, pion etc) We will discover that the electron and photon are indeed fundamental, elementary particles, but protons and neutrons are made of even smaller elementary particles called quarks Cosmic Rays CLASSIFICATON OF PARTICLES An elementary particle is a point particle without structure that is not constructed from more elementary entities With the advent of particle accelerator in the 1950’s many new elementary particles were discovered. The question arose whether perhaps there were too many to all be elementary. This has led to the need for classification of particles. Year of discovery of the particles… FUNDAMENTAL INTERACTIONS AND THE CLASSIFICATION OF PARTICLES Fundamental interactions 1. 2. 3. 4. gravitation electromagnetic strong nuclear force weak nuclear force Participating particles 1. 2. 3. 4. all particles with mass those carrying charge Hadrons (and quarks) Leptons (and quarks) HADRONS Hadrons interact through strong forces. There are two classes, mesons and baryons. Mesons have zero or integral spin (0 or 1) with masses that lie between the electron and the proton. Baryons have half integral spin (1/2 or 3/2) and have masses that are always greater than or equal to that of the proton. Hadrons are not elementary particles. As we will see later, they are made of quarks LEPTONS Leptons interact through weak interactions, but not via the strong force. All leptons have spin of 1/2. There are six kinds of lepton: electron e-, muon m-, and tau t -, and 3 neutrinos ne, nm, nt Note that each distinct neutrino is associated with one of the other leptons Leptons were originally named because they were “Light-particles”, but we now know the Tau is twice as heavy as a proton Neutrinos were originally thought to be massless, but they probably have a small mass Read more in Tipler p. 1336 Matter & Antimatter Every particle has an antiparticle partner Here are some examples e- - electron p - proton e+ - positron p - antiproton n - neutron n - antineutron n - neutrino n - antineutrino Antimatter For each particle there is an associated antiparticle Anti-particles always created in particle-anti particle pairs s Electron Pair Production g -> e- + e+ s Eg 2 x 511 keV e- e+ * Antiparticle has the same mass and magnitude of spin as the particle * Antiparticle has the opposite charge to the particle * The positron is stable but has a short-term existence because our Universe has a large supply of electrons * The fate of a positron is annihilation Some Fundamental Particles Spin Antiparticle Rest energy MeV Charge g 0 0 1 g Leptons Neutrino Electron Muon n em- 0 0.511 105.7 0 -1 -1 1/2 1/2 1/2 n e m Meson Pion o 140 135 +1 0 0 0 o Baryons Proton neutron p+ no 938.3 939.6 +1 0 1/2 1/2 p- Mass less boson photon - Particle Symbol n The Conservation Laws Can a conceivable reaction or decay occur? • Conservation of Lepton number contd: …..because the neutrino associated with an electron is different to a neutrino associated with a muon, we assign separate Lepton numbers Le, Lm and Lt to the particles e.g. for e and ne, Le=+1, for their anti-particles Le=-1, and for all other leptons and other particles Le=0 • Conservation of Strangeness There are other conservation laws which are not universal, e.g. strange particles have a property called strangeness which must be conserved in a decay or reaction Checking Baryon Numbers 2p+ + p + n _ _ p+ + p + p _ a) p+ + n b) p+ + n Answer: a) Baryons = 1+1 on left hand side Baryons = 2 on right hand side too! Allowed reaction! b) Baryons = 2 on left hand side Baryons = -1 on right hand side Forbidden reaction Checking Lepton Numbers a) µb) π+ _ e- + n e + n m µ+ + nm + ne Answer: a) Before decay Le = 0 and Lm = +1 After decay Le = 0 and Lm = +1 Allowed reaction! b) Before decay Lm = 0 and Le = 0 After decay Lm = 0 and Le = 1 Forbidden reaction! Is Strangeness Conserved? a) π+ + n b) π- + p K+ + - + Answer: a) Initial state has S = 0 Final state has S = +1 - 1 = 0 Allowed reaction! b) Initial state has S = 0 Final state has S = -1 Forbidden reaction! Conservation Laws • Test the following decays for violation of the conservation of electric charge, baryon number and lepton number. • (a) n -> - m m• (b) 0 - e+ + e- + g Conservation Laws Solution • Method: Use the table from the formula sheet and the conservation laws for Baryon number and Lepton number • (a) n -> - m m– Total charge on both sides = 0 : conserved – Baryon number changes from +1 to 0: violated – Lm = 0 on both sides : conserved – Process not allowed • (b) 0 - e+ + e- + g – Total charge on both sides = 0 : conserved – Baryon number on both sides = 0 : conserved – Le = 0 on both sides: conserved – Process is allowed Three Different Types of QUARKS There are three elementary quarks (flavors) That make up the fundamental particles: Up u Down d Strange s π+ u d p Baryon Name Up u Down d Strange s Spin Charge 1/2 +2/3 1/2 -1/3 1/2 -1/3 Baryon Strangeness 1/3 0 1/3 0 1/3 -1 Anti-quarks maintain spin, but change sign of S and B! Meson u u d Different types of quarks contd. • Mesons – quark + anti-quark q q • Baryons – three quarks q q q • Anti-baryons – three anti-quarks By 1967 it was realised that new kinds of quarks were required to explain discrepancies between the model and experiment Charm (c) Bottom (b) – discovered 1977 Top (t) – discovered 1995 Quark combinations • Find the baryon number, charge & strangeness of the following quark combinations and identify the hadron: • (a) uud • (b) udd • (c) uus • (d) dds Quark combinations Solution Method: for each quark combination determine the baryon number B, the charge q and the strangeness S; then use Table from formula sheet to find a match. • (a) uud – – – – B = 1/3 + 1/3 + 1/3 = 1 q = 2/3 + 2/3 – 1/3 = 1 S=0 It is a proton • (b) udd – – – – B = 1/3 + 1/3 + 1/3 = 1 q = 2/3 – 1 /3 – 1/ 3 = 0 S=0 It is a neutron • (c) uus – Ditto, B=1, q=1, S= -1 and it is a + • (d) dds – Ditto, B=1, q=-1, S= -1 and it is a - True or false? • (a) Leptons consist of three quarks • (b) Mesons consist of a quark and an anti-quark • (c) The six flavors of quark are up, down, charmed, strange, left and right • (d) Neutrons have no charm (a) False: leptons are fundamental particles e.g e(b) True (c) False: there is no left and right quark, but there are top and bottom quarks (d) True: neutrons are made of udd quarks Quark confinement • No isolated quark has ever been observed • Believed impossible to obtain an isolated quark • If the PE between quarks increases with separation distance, an infinite amount of energy may be required to separate them • When a large amount of energy is added to a quark system, like a nucleon, a quark-antiquark pair is created – Original quarks remain confined in the original system • Because quarks always confined, their mass cannot be accurately known Crib sheet (or what you need to know to pass the exam) • The zoo of particles and their properties – – – – Leptons (e-, m-, -, ne , nm, n) Hadrons (baryons and mesons) Their anti-particles The conservation laws and how to apply them (energy, momentum, baryon number, lepton numbers, strangeness) • Quarks and their properties – – – – Flavors: up, down, strange, charm, top ,bottom How to combine quarks to form baryons and mesons Quark spin and color The eight-fold way patterns • Fundamental forces and field particles • The standard model The Photoelectric Effect What you need to know… • Relationship between the energy of a photon, and the frequency of the photon • The electronvolt • The work function • Photoelectric equation What is the photoelectric effect? • Provides evidence that electromagnetic waves (eg light) have particle like behaviour. • When a metallic surface is exposed to electromagnetic radiation (light) above a certain frequency (called the ‘threshold frequency’) the photons from the light are absorbed and current is produced. • An electron in the metal can absorb the energy from the photon, and if there is enough energy the electron can escape the metal! From experimentation… • There is no emission of electrons below the threshold frequency. • This frequency is different for different metals. • Above the threshold frequency, electrons are emitted. • The kinetic energy of the elcectons can vary. • Their kinetic energy is given as K.E. = 1/2 mv2 Continued… • Increasing the frequency of the radiation, increases the maximum kinetic energy of the electrons. • This however has no effect on the ‘photoelectric current’ which is the rate of emission of electrons. • If you increase the intensity of the radiation (for example by shining more light on the metal), will have no effect if the frequency is still below the threshold. • If the intensity is increased, and the frequency is above the threshold, then you will increase the photo electric current. (more light in = more electrons out) What this means Frequency BELOW Frequency ABOVE Threshold Threshold Increase frequency If frequency still below threshold then nothing. If frequency is above threshold then electrons are emitted Increased kinetic energy of electrons Increase intensity NOTHING Greater photoelectric current Explanation of Photoelectric Effect • Relies on the idea of a photon being a ‘quantum’ of enegy. • What does this mean? • Quantum is another term for packet. • Therefore the photoelectric affect relies on the idea that light is not made up of waves, but that it is made up of particles called photons, that have packets of energy. The relationship between the energy (E) of a photon and its frequency (f) is: E = hf Where h is Plank’s constant which is equal to 6.63x10-34 Js The Electronvolt… This is something that always scares people when they first see it! DON’T PANIC! It is simply a unit used to describe energy (like Joules). I electronvolt (eV) is the amount of energy needed to move 1 electron across a potential difference of 1 volt 1 eV = 1.60x10-19 J Einstein’s Explanation of Photoelectric Emission • An electron needs to absorb a minimum amount of energy to escape from a metal. • This minimum amount is a property of a metal and is called the ‘work function’ () • If the photons hitting the metal have energy (hf) which is less than then no electrons are emitted. • Electrons can be emitted just when hf = . The Work Function cont. • For photons with an energy larger than , the electrons emitted from the metal have a ranges of energies. • The electrons with the largest (or maximum) energy needed the minimum energy to escape. • Increasing the intensity of the radiation increases the number of photons emitted, but does NOT affect the electrons kinetic energy. Einstein’s Photoelectric Equation • This relates the maximum kinetic energy of the emitted electrons to the work function and the energy of each photon: • hf = + Ek • Ek = (1/2 mv2) which is the maximum kinetic energy. • At the threshold frequency, Ek equals zero so hf =