Section B Review
advertisement
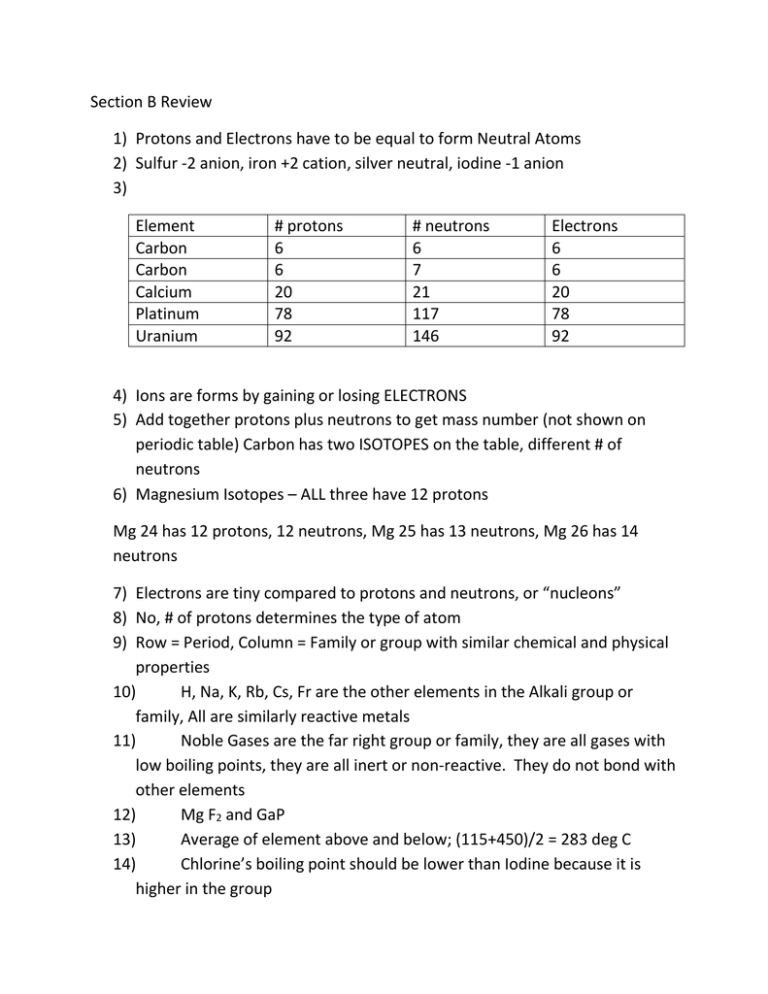
Section B Review 1) Protons and Electrons have to be equal to form Neutral Atoms 2) Sulfur -2 anion, iron +2 cation, silver neutral, iodine -1 anion 3) Element Carbon Carbon Calcium Platinum Uranium # protons 6 6 20 78 92 # neutrons 6 7 21 117 146 Electrons 6 6 20 78 92 4) Ions are forms by gaining or losing ELECTRONS 5) Add together protons plus neutrons to get mass number (not shown on periodic table) Carbon has two ISOTOPES on the table, different # of neutrons 6) Magnesium Isotopes – ALL three have 12 protons Mg 24 has 12 protons, 12 neutrons, Mg 25 has 13 neutrons, Mg 26 has 14 neutrons 7) Electrons are tiny compared to protons and neutrons, or “nucleons” 8) No, # of protons determines the type of atom 9) Row = Period, Column = Family or group with similar chemical and physical properties 10) H, Na, K, Rb, Cs, Fr are the other elements in the Alkali group or family, All are similarly reactive metals 11) Noble Gases are the far right group or family, they are all gases with low boiling points, they are all inert or non-reactive. They do not bond with other elements 12) Mg F2 and GaP 13) Average of element above and below; (115+450)/2 = 283 deg C 14) Chlorine’s boiling point should be lower than Iodine because it is higher in the group 15) Metals (left side of periodic table) tend to lose electrons and become positive 16) Positive Cations = Sodium, lithium, calcium, copper, tin, Negative anions = fluorine, oxygen, iodine 17) Noble gases rarely exchange electrons and rarely bond with other atoms 18) A) anion B) neutral C) neutral D) cation E) cation 19) A) gain B) none C) none D) lose E) lose 20) H , Na 1+, Cl 1-, Al 3+ 21) A) Potassium Iodide B) Calcium Sulfide C)Iron (III) Bromide D) Barium Hydroxide E) Ammonium Phosphate F) Aluminum (III)oxide 22) The periodic table helped scientists organize the known properties of elements and as a tool to help them predict the nature of undiscovered items. 23) Bar graphs represent discontinuous data because they do not show information between known data 24) Data Tables a. Before lab – helps organize data collection b. During lab – organize collection of data c. After Lab – assist with analyzing trends in data 25) The ion models represented the number of charges that needed to be matched between cations and anions to form neutral compounds. The models are not perfect because they do not represent other ion properties. 26) Calcium is more likely to react with a chromium (III) chloride solution because Calcium is more reactive than chromium 27) Zn is more reactive than silver, so zinc(s) solid will replace Ag(aq) in solution (Reaction B) 28) An iron spoon is more reactive than Lead , so an iron spoon would dissolve and replace the lead in solution. Fe + PbNO3 FeNO3+ Pb 29) Oxygen isotopes would be more similar chemically (same electron configurations) 30) Average the properties of the element above and below; 205 picometers 31) A) Bromine B) Silicon 32) Many answers; Ar and K 33) Magnesium is more reactive than Iron and aluminum common building materials 34 and 35 are not required. Aluminum corrodes until a thin coating of Aluminum Oxide is formed and then it doesn’t react anymore. Iron will continue to corrode until the iron is gone.


