Oxidation-Reduction Reactions
advertisement

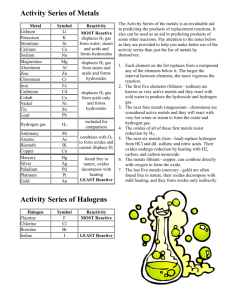
Predicting Products of Reactions AP Chemistry Ms. Paskowski More reactive element will replace a less reactive element Metals – use the activity series or the Standard reduction tables Nonmetals (easier) – use the activity table The more reactive metal will replace the less reactive metal within a compound Example • Zn (s) + CuSO4 Cu (s) + ZnSO4 The more reactive zinc replaces copper. STANDARD REDUCTION POTENTIALS All reactions listed are reduction reactions. The reactions most likely to occur have the largest positive value. The more positive, the more likely to be reduced M + AB or N + CD where M = metal and N = Nonmetal Use Activity table to determine whether • M is more active than A or less likely to be reduced, if so a reaction will occur. • N is more active than D or more likely to be reduced, if so a reaction will occur Chemistry: Matter and Its Changes • Page 224 #5.50 and 5.51 Very active metals will combine with water to form bases • Hydroxides • Often produce hydrogen gas Very Reactive Nonmetals will react with water to form acids • Conditions will dictate which acid is formed. Nonoxidizing acids– i.e. HCl, HBr • Hydrogen will be replaced by a more active metal. H will be reduced and the metal will be oxidized. Anion does not participate Oxidizing Acids always contain oxygen. • Anion is sometimes a stronger oxidizer than H+ • i.e. HNO3, H2SO4 (under certain conditions) Acids will react with active metals Using the Standard Reduction Potential table • If the reduction value is negative , the metal will replace the H in the acid and Hydrogen gas will be produced. • If the reduction value is positive, no reaction will occur. For anoxy acids, the products are a salt and hydrogen gas • M + HA MA + H2 For oxoacids, the products are a salt, water, and a gas • M + HAxOy H2O + M+ + AzOq Practice Problems • Page 224 # 46-49 Oxidizer! • Combustion is the adding of oxygen to carbon and hydrogen (and sulfur and nitrogen). Reaction Reaction with metals form oxides with nonmetals produce nonmetallic oxides Soluble Metallic oxides • MxOy produce bases (hydroxides) when dissolved in water Example NaOH + H2O Na+ + OH- Nonmetallic oxides • Produce acids when dissolved in water • Example N2O5 + H2O H+ + NO3 – Metallic oxides plus acid • Produce a salt and water Metallic oxides are weak bases and will react with the acid to produce salt and water Nonmetallic oxides plus bases • Nonmetallic oxides are acidic • Produce a salt and water Nonmetallic oxides are weak acids and will react with the base to produce salt and water Metallic oxides and nonmetallic oxides will combine to form a salt Example • MgO + CO2 MgCO3 • CaO + SO3 CaSO4 Metals plus nonmetals • Adding Hydrogen makes hydrides • Adding halogens makes halides • Adding nitrogen makes nitrides • Adding sulfur makes sulfides • Adding oxygen makes oxides Adding LOTS of oxygen makes peroxides • Adding water makes hydroxides or oxides and hydrogen gas Problems given will usually produce a oxygen or water Examples • CaCO3 CaO + CO2 • H2O2 H2O + O2 • KClO3 KCl + O2 • Al(OH)3 Al2O3 + H2O 1) 2) Write all the species down in their physical state, i.e. liquid, solid, gas, or ions in solution Determine the type of reaction that could occur 1) Double replacement – acid/base or precipitate 2) Oxidation-Reduction – single replacement, combustion, decomposition (only one reactant), synthesis 3) If nothing else fits, use a simple redox reaction where the charges on the ions change.