Atoms and Bonding Study Guide
advertisement

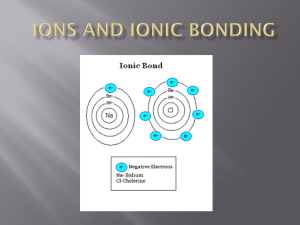
Name: _____________________________________________ Period: ______ Atoms and bonding study guide 1) What is the force that holds atoms together? 2) Why do atoms form chemical bonds? 3) Name the following compounds: K2O, NaF, PI3 4) What are the subatomic particles found in the outermost energy level of an atom? 5) How many valence electrons do most atoms need to be stable? _____ 6) Which elements only require 2 valence electrons to be stable? 7) Draw an electron dot diagram for: He, Li, C, Ca, Al, Si, P, O, Cl 8) Which of the above elements are likely to bond with one another (put them in pairs, one will be left out)? 9) Write the charges for the following ions: H, Li, K, Cl, Si 10) Write the correct chemical formula for the following covalent compounds: Dihydrogen disulfide, Carbon disulfide, Carbon tetraiodide 11) Write the correct chemical formula for the following ionic compounds: Magnesium nitride, Sodium chloride, Silicon oxide