Lab Handout Set G
advertisement
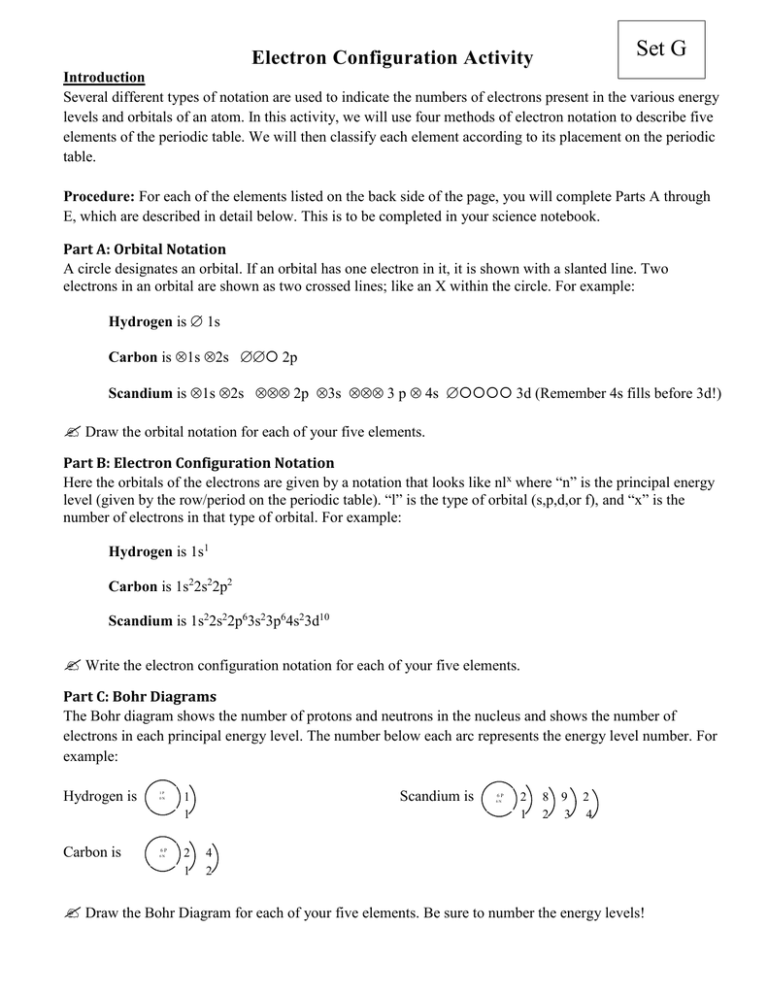
Set G Electron Configuration Activity Introduction Several different types of notation are used to indicate the numbers of electrons present in the various energy levels and orbitals of an atom. In this activity, we will use four methods of electron notation to describe five elements of the periodic table. We will then classify each element according to its placement on the periodic table. Procedure: For each of the elements listed on the back side of the page, you will complete Parts A through E, which are described in detail below. This is to be completed in your science notebook. Part A: Orbital Notation A circle designates an orbital. If an orbital has one electron in it, it is shown with a slanted line. Two electrons in an orbital are shown as two crossed lines; like an X within the circle. For example: Hydrogen is 1s Carbon is 1s 2s 2p Scandium is 1s 2s 2p 3s 3 p 4s 3d (Remember 4s fills before 3d!) Draw the orbital notation for each of your five elements. Part B: Electron Configuration Notation Here the orbitals of the electrons are given by a notation that looks like nlx where “n” is the principal energy level (given by the row/period on the periodic table). “l” is the type of orbital (s,p,d,or f), and “x” is the number of electrons in that type of orbital. For example: Hydrogen is 1s1 Carbon is 1s22s22p2 Scandium is 1s22s22p63s23p64s23d10 Write the electron configuration notation for each of your five elements. Part C: Bohr Diagrams The Bohr diagram shows the number of protons and neutrons in the nucleus and shows the number of electrons in each principal energy level. The number below each arc represents the energy level number. For example: Hydrogen is Carbon is 1P 0N 1 1 6P 2 1 6N Scandium is 6P 6N 2 1 8 2 9 3 2 4 4 2 Draw the Bohr Diagram for each of your five elements. Be sure to number the energy levels! Part D: Lewis Dot Diagrams/Electron Dot (p. 229 in your textbook.) Most atoms form chemical bonds using only their outer electrons. They share, or transfer electrons in order to attain a stable “octet” or eight electrons in their outer shell. Electron dot diagrams show dots only for the electrons in the outermost energy level. Each electron is represented by a dot grouped around the symbol of the atom. Two dots together represent a pair of electrons in an orbital. For example: Hydrogen is: Carbon is: Scandium is: Draw the Lewis Dot Diagram for each of your five elements. Why will you never have more than 8 dots? Part E: Classification of Elements (p. 171-172 in your textbook.) There are three main categories, and some families, of elements. Elements in the same category have similar chemical properties and similar locations on the periodic table. Representative Elements are in the two families at the left of the periodic table and the five families to the left of the Noble gases (Groups 1A to 7A but not the B groups.). Several families of the representative elements have special names. Group 1A is called the alkali metals. Group 2A is the alkaline earth metals, and group 7A is called the halogens. Noble Gases are group 8A, the far right-hand family on the periodic table. They do not readily form chemical compounds, thus they are also called the “inert gasses.” Transition Metals are the ten center families; the part that looks like a dog took a bite out of the top of the table. These are groups 1B to 10B. Inner Transition Metals are the two rows at the bottom of the periodic table, separated from the rest of the elements. Classify each of your five elements. If it is classified as a Representative element and it has a special family name, you are to write both labels! For example, “Lithium is a representative element in the family alkali metals.” Postlab question: Explain. Why does the actual electron configurations for some elements differ from those assigned using the Aufbau principle? [Hint: Information on p. 137 of the textbook might help.] Set G Elements 19 9F 35 17Cl 84 36Kr 133 55Cs 64 29Cu