The Periodic Table

Organization and Features
Mendeleev organized the elements based on increasing atomic mass.
Moseley later organized by atomic number.
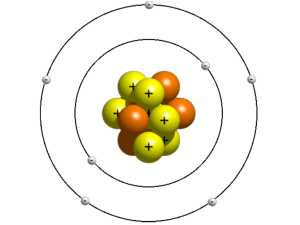
• Atomic number# of protons in an element
(equivalent to the # of electrons)
The periodic table is divided into 8 main groups not including the transition metals in the middle.
•
• Groups- vertical columns & contain elements with similar chemical properties.
Groups also have the same number of electrons in the outer shell.
Valence Electrons
Some of these groups have special names based on the properties that they exhibit.
•
•
•
• Group 1 – Alkali metals
Group 2 – Alkaline Earth metals
Group 7 – Halogens
Group 8 – Noble Gases
The noble gases are a group of very unreactive elements.
Why do you think that we use Helium to inflate things?
Has anyone ever heard of the Hindenburg?
Periods- Rows that go across the periodic table from left to right.
•
• As you move left to right on the periodic table, the atomic masses of the elements increases.
Atoms on the left of the period are usually larger and lighter than the smaller, heavier atoms on the right of the period.
The periodic tables contains a stair step line.
Separates elements as metals, nonmetals, and metalloids.
PHYSICAL PROPERTIES
Show luster (shininess)
Good conductors of heat and electricity
High density
High melting point
Ductile-most metals can be drawn out into thin wires
Malleable- can be hammered into thin sheets
CHEMICAL PROPERTIES
Easily lose their electrons
Corrode easily: a gradual wearing away
• Ie. Silver tarnishing or iron rusting
PHYSICAL PROPERTIES
No luster- dull appearance
Poor conductors of heat and electricity
Brittle when solid-breaks easily
Not ductile
Not malleable
Low density
Low melting point
CHEMICAL PROPERTIES
Tend to gain and share electrons
Solids
Can be shiny or dull
Ductile
Malleable
Conduct heat and electricity better than nonmetals but not as well as metals