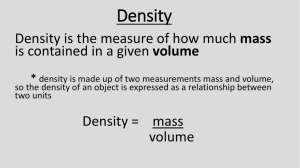CH. 2 Test Review
advertisement

CH. 2 Test Review Chemistry I Object I 1. Measure the mass of Object I. a. Record mass in grams. b. Use dimensional analysis to convert grams to kg. c. On your paper: a. grams_____ b. ______g_|_______ = ______kg | Object I 2. Measure the volume of Object I. a. Record volume in cm3. b. What is the volume of this object in mL? Hint: See your conversion chart. c. On your paper: a. cm3_____ b. ______mL 3. Calculate the density of Object I. a. Write the formula for density. b. Use the measurements from the previous two questions (grams and mL) to find density. c. On your paper: a. Density formula: D = b. Density of Object I = (Include units!) 4. Units of length: km, cm, mm, m a. Rank the units above in order from smallest to largest. ____, ____, _____, _____ b. Rank the units above in order from largest to smallest. ____, ____, _____, _____ c. Identify which unit is most appropriate for measuring: (1) football field ______, (2) a book____, (3) a truck_____ d. SI unit for length is_____. 5. Units of mass: kilogram, milligram, gram a. Rank the units above in order from smallest to largest. ____, ____, _____ b. Rank the units above in order from largest to smallest. ____, ____, _____ c. Identify which unit is most appropriate for measuring: (1) a football player ______, (2) a book____, (3) a medicinal pill_____ d. SI unit for mass is_____. 6. Metric Units a. Two units for density: ___ and ____ b. The prefix centi- means ______ c. The prefix kilo- means ______ d. The prefix milli- means_____ 7. Scientific Method a) The validity of scientific concepts is evaluated by testing ____________________. b) If you think data is badly collected, it is OK to ignore that data. True or False?_____ 8. Significant Figures a. The number of significant figures in 4 x 1023 is __. b. The number of significant figures in 0.00305 is ____. c. Four thousand thirteen expressed to ONE sig fig is _____. 9. Show dimensional analysis to solve. e. 0.25 g is equivalent to ______________ kg. f. 56 lb = _______ kg 10. Scientific Notation a. When 6.02 x 1023 is multiplied by 9.1 x 10–31, the product is _____________. b. Write 0.0055 in scientific notation________ c. Write 6 550 000 in scientific notation________ 11. Accuracy and Precision The data below correspond to the measurement of a marble with a known density of 3.2 g/mL by four individuals. Which observations are most precise? Which are most accurate? a. Most precise______ b. Most accurate________ Batman Robin The Joker Catwoman 3.2 g/mL 3.2 g/mL 2.5 g/mL 2.5 g/mL 3.7 g/mL 3.3 g/mL 2.4 g/mL 3.7 g/mL 3.0 g/mL 3.2 g/mL 2.4 g/mL 3.0 g/mL 3.4 g/mL 3.4 g/mL 2.6 g/mL 4.0 g/mL 12. Accuracy and Precision Examine the dart board. Are the darts on the dartboard accurate, precise, or both? Explain. 13. Mass = _____ = _____g The density of aluminum is 3.70 g/cm3. The volume of a solid piece of aluminum is 2.50 cm3. Find its mass. Hint: First write the formula from the density equation. 14. Quantitative vs. Qualitative Label each of the observations shown as qualitative or quantitative a. The solution is blue.____ b. There are 125 mL in the flask.______ c. The solution temperature is 45° C._____ d. The solution is translucent. 9. Accuracy and Precision Examine the dart board. Which darts are most precise? Which are most accurate? a. Most precise______ b. Most accurate________