Density
advertisement

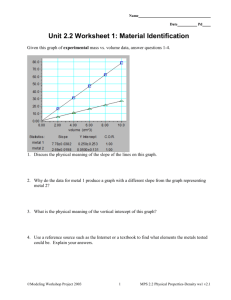
Density Read through the PPT and take notes on Density. Don’t forget a Title and a subtitle for each section! Hint: Titles are underlined & Important things are in red. What is density? Density is a comparison of how much matter there is in a certain amount of space. Density is the measure of the “compactness” of a material. Density is determined by how close the atoms are to each other “Compactness” All substances have density including liquids, solids, and gases Density is a RATIO of mass to volume. If the temperature stays the same, the Density of solids & liquids do not change… discuss this. Because of this density is a Characteristic Property and is used to help identify unknown substances. If you take the same volume of different substances, then they will weigh different amounts. Wood Water Iron 1 cm3 1 cm3 1 cm3 0.50 g 1.00 g 8.00 g IRON Q) Which has the greatest mass and therefore the most dense? Which one is more dense? Demonstration: Hula-hoop Density… try it! How about this: Which square is more dense? Why? If the volume is the same but the mass is higher then density will be higher - More matter in the same space! Illustrate and label the two different densities as more & less dense. Which one is more dense? Now which one is more dense? Why? If the mass is the same but the volume is smaller . . . The density will be higher. Same amount of matter but a smaller volume or space! Illustrate and label the two different densities as high & low. How do we determine density? To calculate the density of something: Density = mass ÷ volume OR Units for density: g cm3 Why are these the units for density? mass OR m volume v ALWAYS REMEMBER UNITS! A little trick to remember! I DENSITY Let’s try a density problem together Frank has a paper clip. It has a mass of 9g and a volume of 3cm3. What is its density? M g D= V D = cm3 D = 39cmg 3 D = 3 cmg 3 Frank also has an eraser. It has a mass of 3g, and a volume of 1cm3. What is its density? 3g g M D = V D = cm3 D = 1cm3 D = 3 cmg 3 Work on these problems with your neighbor. Jack has a rock. The rock has a mass of 6g and a volume of 3cm3. What is the density of the rock? Jill has a gel pen. The gel pen has a mass of 8g and a volume of 2cm3. What is the density of the pen? Now, try these on your own. Al’Licia has a watch. It has a mass of 4g and a volume of 2cm3. What is the density of the watch? Mia has a wallet. It has a mass of 15g and a volume of 5cm3. What is the density of the wallet? Think about it… Do water and ice have the same density? Proof that ice and water have DIFFERENT densities! What would happen???? Mercury density = 13,600 kg/m3 Lead density = 11,340 kg/m3 Lead floats on liquid mercury! Liquid Layers If you pour together liquids that don’t mix and have different densities, they will form liquid layers. Can you think of examples? Let’s Do a Demonstration: Density Column The liquid with the lowest density (NOT very dense) will be on the top or float.. The liquid with the highest density (more dense) will be on the bottom or sink. Liquid Layers Check out this picture from your book. Which layer has the highest density? Which layer has the lowest density? Imagine that the liquids have the following densities (remember liquid volume is mL or L): 10 g/mL 3 g/mL 6 g/mL 5 g/mL Which density would belong with which layer? Liquid Layers – Try with your neighbor Which liquid has the highest density? Which liquid has the lowest density? Which liquid has the middle density? Liquid Layers – Try on your own! Imagine that the liquids on the right have the following densities: 15 g/mL 3 g/mL 7 g/mL 10 g/mL 9 g/mL 12 g/mL Match the colors to the correct densities. 3g/mL 7g/mL 9g/mL 10g/mL 12g/mL 15g/mL Review What is the formula for density? What happens if you pour together liquids that have different densities? Will the liquid on the top have the highest or lowest density? Will the liquid on the bottom have the highest or lowest density? Super Scientist Question of the Day Jake has a book, a ruler, and a balance. Explain how can Jake find the density of the book with the tools he has?