ATOMIC FORCE MICROSCOPY
advertisement

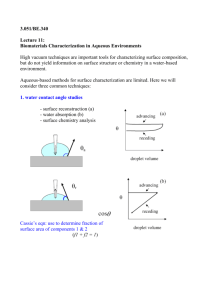
HIGH-RESOLUTION IMAGING WITH FORCES (ATOMIC FORCE MICROSCOPY) Krystyn J. Van Vliet krystyn@mit.edu 3.052 Spring 2003 March 4, 2003 Review: Typical HRFS output on stiff substrate compression no interaction CONVERTED DATA repulsive regime jump-to-contact 0 adhesion 0 z-Piezo Deflection, z (nm) kc attractive regime 0 Tip-Sample Separation Distance, D (nm) Force, F (nN) Photodiode Sensor Output, s (V) RAW DATA substrate Review: Typical HRFS output on stiff nm) repulsive regime kc attractive regime 0 Tip-Sample Separation Distance, D (nm) Force, F (nN) no action CONVERTED DATA substrate Review: Typical HRFS output on stiff substrate compression no interaction CONVERTED DATA repulsive regime jump-to-contact 0 adhesion 0 z-Piezo Deflection, z (nm) kc attractive regime 0 Tip-Sample Separation Distance, D (nm) Force, F (nN) Photodiode Sensor Output, s (V) RAW DATA substrate Review: Experimental Aspects of Force Spectroscopy Conversion of raw data in a high-resolution force spectroscopy experiment : • sensor output, s transducer displacement, d force, F F=kd d = s/m • z-piezo deflection, z tip-sample separation distance, D D=zd Typical force spectroscopy data for a weak cantilever on stiff substrate (ksample>> kcantilever) : RAW DATA substrate compression D no interaction D jump-to-contact B/C 0 E F adhesion CONVERTED DATA D D repulsive regime A B/C G kc E attractive regime A G F 0 0 z-Piezo Deflection, z (nm) Tip-Sample Separation Distance, D (nm) *Note: For an adhesive interaction Force, F (nN) cantilever undeflected, zero force (set F=0) • B/C: attractive interaction pulls tip down to surface and tip jumps to contact, cantilever exhibits mechanical instability • D: contact, constant compliance regime, no sample indentation, tip and sample move in unison (Ds/Dz=1) RETRACT :(*sample and tip move apart) • D: repulsive contact, constant compliance regime, tip deflected up • E: attractive force (adhesion) keep tip attached to surface, tip deflected down • F: tip pulls off from surface, cantilever instability • G: same as region A Photodiode Sensor Output, s (V) APPROACH : (*sample and tip come together) • A: tip and sample out of contact, no interaction, Atomic Force Microscopy Imaging • BASIC PRINCIPLES : piezo rasters or scans in x/y direction across sample surface cantilever deflects in response to a topographical feature computer adjusts the z-piezo distance to keep the cantilever deflection constant and equal to the setpoint value “feedback loop” : system continuously changes in response to an experimental output (cantilever deflection) ERROR SIGNAL = actual signal- set point (*used to produce 2D topographical image in contact mode) Atomic Force Microscopy: General components and functions cantilever AFM : Normal Force Spectroscopy Modes of Operation *AC=dynamic(tip is driven to oscillate), DC=static(no external oscillation on tip) Contact (DC and AC) : Force Modulation Intermittent Contact : Tapping (AC) Non-Contact (AC) AFM : Contact Mode Output: “Isoforce” Height Feedback Error: Deflection http://www.physik3.gwdg.de/~radmacher/publications/osteoblasts.html AFM : Tapping Mode Output: “Isoamplitude” Height Feedback Error: Amplitude Additional Feedback: Phase Evaporated gold surface AFM : Normal Force Spectroscopy Modes of Operation AFM : First high resolution images LAYERED HARD CRYSTALLINE SOLID MATERIALS Highly Oriented Pyrolytic Graphite (HOPG) SAMPLE graphite molybdenum sulfide boron nitride gold sodium chloride (001) lithium flouride (1014) cleavage plane of a calcite (CaCO3) crystal REFERENCE Binnig, et al., Europhys. Lett. 3, 1281 (1987) Albrecht, et al., J. Vac. Sci. Tech. A 6 271 (1988) Manne, et al., Appl. Phys. Lett. 56 1758 (1990) Meyer, et al., Appl. Phys. Lett. 56 2100 (1990) Meyer, et al., Z. Phys.B. 79 3 (1990) Ohnesorge, et al., Science 260 1451 (1993) (http://www.energosystems.ru/fgallery.htm) http://stm2.nrl.navy.mil/how-afm/how-afm.html http://www.physics.sfasu.edu/afm/afm.htm AFM: Tip Functionalization 1. Gold coating Purpose: Methods: TOP VIEW TOP VIEW Si3N4 cantilever ONE-TIME Au COATING : 100 nm heterogeneous, rougher larger polydomain microstructure SIDE VIEW INTERVAL Au COATING : Si chip homogeneous, smoother smaller polydomain microstructure 100 nm SIDE VIEW AFM: Tip Functionalization 2. Chemical coating http://www.di.com/AppNotes/LatChem/LatChemMain.html Purpose: microfabricated Si3N4 probe tip Methods: • Molecular Elasticity of Individual Polymer Chains • Protein Folding • DNA Interatomic Bonds • Receptor-Ligand Interactions • Covalent Bonds • Colloidal forces Applications: • Van der Waals forces • Hydration forces • Hydrophobic forces • Surface Adhesion • Nanoindentation • Electrostatic DLVO forces • Cell Adhesion • Steric Forces of Polymer Brushes + - + synthetic polymers - - - - + - proteins polyelectrolytes + - - antibodies - - + + self-assembling monolayer ligands AFM: Tip Functionalization (c) (c) colloidal particle (a) Single Cell Dictyostelium Discoideum (d) nanotube with individual ligand (b) E. Coli Bacteria (a) Benoit, M.; Gabriel, D.; Gerisch, G.; Gaub, H. E. Nature Cell. Bio 2000, 2 (6), 313. (b) Ong, Y-L.; Razatos, A.; Georgiou, G.; Sharma, M. K. Langmuir 1999, 15, 2719. (c) J . Seog, Ortiz/ Grodzinsky Labs 2001 (d) Wong S.S.; Joselevich E.; Woolley, A.T.; Cheung, C. L.; Lieber, C. M. Nature 1998, 394 (6688), 52. AFM: Applications of modes Timeline: Contact DC and AC (Force Modulation Microscopy (FMM), Phase Imaging): Hansma, et al., 1991 Intermittant Contact/Tapping / Lift (AC): Hansma, et al., 1994 Noncontact (NC) 1995 I. Normal Force Microscopy II. Friction or Lateral Force Microscopy (FFM/ LFM) Frisbie, et al., 1994 III. Force / Volume Adhesion Microscopy Radmacher, et al., 1994 http://www.di.com/AppNotes/ForceVol/FV.array.html X X X X X X XX X XX XX X X X X X X X X=-OH,-CH3, -NH2 IV. Chemical Force Microscopy (CFM) Frisbie, et al., 1994 Surface Maps: Topography & Roughness, Electrostatic Interactions, Friction Chemical, Adhesion , Hardness, Elasticity /Viscoelasticity Dynamic Processes : Erosion, Degradation, Protein-DNA Interactions AFM: Resolution factors/Artifact sources Physik Instruments, Nanopositioning 1998 SPECIMEN DEFORMATION & THERMAL FLUCTUATIONS Hoh, et al. Biophys. J. 1998, 75, 1076. D+DD d -Z D L+DL ~ +Z L voltage applied -X +Y +X ADHESION FORCE Yang, et al. Ultramicroscopy 1993, 50, 157 Force, F (nN) PIEZO AMPLIFIER, SENSOR AND CONTROL ELECTRONICS, MECHANICAL PARAMETERS y Distance, D (nm) x connecting wires polarization PROBE TIP SHARPNESS CANTILEVER THERMAL NOISE Lindsay Scanning Tunneling Microscopy and Spectroscopy 1993, 335. kt Shao, et al. Ultramicroscopy 1996, = 66, 141. cantilever Sheng, et al. J. Microscopy 1999, 196, 1. m dt(max) dt(max) Fadhesion 0 z electrodes 0 m m (*http://cnst.rice.edu/pics.html Lieber, et al., 2000) AFM: Advantages as tool to assess biological responses Biological Applications: AFM Images of Cells Contact mode image of human red blood cells - note cytoskeleton is visible. blood obtained from Johathan Ashmore, Professor of Physiology University College, London. A false color table has been used here, as professorial blood is in fact blue. 15µm scan courtesy M. Miles and J. Ashmore, University of Bristol, U.K. Red Blood Cells Shao, et al., : http://www.people.virginia.edu/~js6s/zsfig/random.html Rat Embryo Fibroblast(*M. Stolz,C. Radmacher, et al., Cardiac Cells Schoenenberger, M.E. Müller Institute, http://www.physik3.gwdg.de/~radmacher/ Biozentrum, Basel Switzerland) Height image of endothelial cells taking in fluid using Contact Mode AFM. 65 µm scan courtesy J. Struckmeier, S. Hohlbauch, P. Fowler, Digital Intruments/Veeco Metrology, Santa Barbara, USA. Biological Applications: Manipulation of Living Cells • rest cantilever on top of cell and monitor cantilever deflection up and down = beating of cell d>0 d=0 d<0 rest position cardiac cell • I. confluent layer of cells : beat regularly in terms of frequency and amplitude, enormous stability of pulsing, cell are synchronized and coupled together : diverse pulse shapes due to macroscopic moving centers of contraction and relaxation • II. individual cell : sequences of high mechanical activity alternate with times of quietness, irregular beating which often last for minutes, active sequences were irregular in frequency and amplitude • III. group of cells: “pulse mapping” Biological Applications: AFM Images of DNA TappingMode image of nucleosomal DNA was the highlight Image of PtyrTlac supercoiled DNA. 750 nm of the "Practical Course on Atomic Force Microscopy in scan courtesy C. Tolksdorf, Digital Biology," held at the Biozentrum in Basel, Switzerland, July Instruments/Veeco, Santa Barbara, USA, and R. 1998. Image courtesy of Y. Lyubchenko. Schneider and G. Muskhelishvili, Istitut für Genetik und Mikrobiologie, Germany. http://www.people.virginia.edu/~js6s/zsfig/DNA.html AFM image of short DNA fragment with RNA The high resolution of the SPM is able to discern polymerase molecule bound to transcription very subtle features such as these two linear dsDNA recognition site. 238nm scan size. Courtesy of molecules overlapping each other. 155nm scan. Bustamante Lab, Chemistry Department, University Image courtesy of W. Blaine Stine of Oregon, Eugene OR AFM: From Nano to MicroStructures Human hair (C. Ortiz) Eggshell