Models of Biodegradation
advertisement

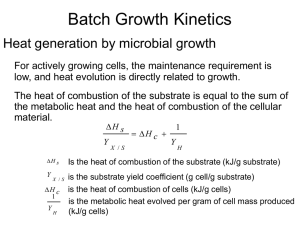
Numerical Modeling of Biodegradation Analytical and Numerical Methods By Philip B. Bedient Modeling Biodegradation • Three main methods for modeling biodegradation Monod kinetics First-order decay Instantaneous reaction • Methods can be used where appropriate for aerobic, anaerobic, hydrocarbon, or chlorinated • Region 1: Lag phase Microbial Growth microbes are adjusting to the new substrate (food source) • Region 2 Exponential growth phase, microbes have acclimated to the conditions • Region 3 Stationary phase, limiting substrate or electron acceptor limits the growth rate log [X] 1 2 3 • Region 4 Decay phase, substrate supply has been exhausted Time 4 Monod Kinetics • The rate of biodegradation or biotransformation is generally the focus of environmental studies • Microbial growth and substrate consumption rates have often been described using ‘Monod kinetics’ dC max CM t dt KC C • • • • C is the substrate concentration [mg/L] Mt is the biomass concentration [mg/ L] µmax is the maximum substrate utilization rate [sec-1] KC is the half-saturation coefficient [mg/L] Monod Kinetics • First-order region, C << KC the equation can be approximated by exponential decay –dC (C = C0e–kt) dt • Center region, Monod kinetics must be used • Zero-order region, C >> KC, the equation can be approximated by linear decay (C = C0 – kt) dC max Mt dt Zero-order region Firstorder region C dC kCMt dt KC Modeling Monod Kinetics • Reduction of concentration expressed as: C C Mt max t Kc C • • • • • Mt = total microbial concentration µmax = maximum contaminant utilization rate per mass of microorganisms KC = contaminant half-saturation constant ∆t = model time step size C = concentration of contaminant Bioplume II Equation - Monod • Including the previous equation for reaction results in this advection-dispersion-reaction equation: C C C C Dx 2 v Mt max t x x Kc C 2 Multi-Species Monod Kinetics • For multiple species, one must track the species together, and the rate is dependent on the concentrations of both species C O C Mt max t Kc C Ko O C O O Mt max F t Kc CKo O Multi-Species • Adding these equations to the advectiondispersion equation results in one equation for each component (including microbes) C O C 1 = (DC vC) Mt max t Rc Rc Kc C Ko O C O O = (DO vO) Mt max F K O t K C c o C O k cY (OC) = (DMs - vMs ) M smax Y bM s t Rm Rm Kc C Ko O M s 1 • BIOPLUME III doesn’t model microbes Modeling First-Order Decay • Cn+1 = Cn e–k∆t • Generally assumes nothing about limiting substrates or electron acceptors • Degradation rate is proportional to the concentration • Generally used as a fitting parameter, encompassing a number of uncertain parameters • BIOPLUME III can limit first-order decay to the available electron acceptors (this option has bugs) Modeling Instantaneous Biodegradation • Excess Hydrocarbon: Hn > On/F • On+1 = 0 • Excess Oxygen: • On+1 = On - HnF Hn+1 = Hn - On/F Hn < On/F Hn+1 = 0 • All available substrate is biodegraded, limited only by the availability of terminal electron acceptors • First used in BIOPLUME II - 1987 Sequential Electron Acceptor Models • Newer models, such as BIOPLUME III, RT3D, and SEAM3D allow a sequential process - 1998 • After O2 is depleted, begin using NO3– • Continue down the list in this order O2 ––> NO3– ––> Fe3+ ––> SO42– ––> CO2 Superposition of Components • Electron donor and acceptor are each modeled separately (advection/dispersion/sorption) • The reaction step is performed on the resulting plumes • Each cell is treated independently • Technique is called Operator Splitting Principle of Superposition Initial Hydrocarbon Concentration + Reduced Hydrocarbon Concentration = Background D.O. Oxygen Depletion Reduced Oxygen Concentration Oxygen Utilization of Substrates • Benzene: C6H6 + 7.5O2 ––> 6CO2 + 3H2O • Stoichiometric ratio (F) of oxygen to benzene 7.5 molO 2 32 mgO 2 1 molC 6 H6 F 1 molC 6 H 6 1 molO 2 (12 6 1 6) mgC 6 H 6 F 3.07 mgO 2 mgC 6 H 6 • Each mg/L of benzene consumes 3.07 mg/L of O2 Biodegradation in BIOPLUME II A A' H Without Oxygen With Oxygen Zone of Treatment Zone of Reduced Hydrocarbon Concentrations B Background D.O. B' A A' D.O. Background D.O. Depleted Oxygen Zone of Oxygen Depletion Zone of Reduced Oxygen Concentration B B' Initial Contaminant Plume Concentration xx x oo o 1.00e + 3 8.89e + 2 7.78e + 2 6.67e + 2 2.22e + 2 1.11e + 2 0.00e + 0 x Injection Well o Production Well Values represent upper limits for corresponding color. Model Parameters Grid Size 20 x 20 cells Cell Size 50 ft x 50 ft Transmissivity 0.002 ft2/sec Thickness 10 ft Hydraulic Gradient .001 ft/ft Longitud inal Dispersivity 10 ft Transverse Dispersivity 3 ft Effective Porosity 0.3 Biodegrading Plume 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 4 7 9 9 5 2 0 0 0 0 0 0 0 0 0 0 0 0 1 6 38 71 97 104 90 54 19 4 1 0 0 0 0 0 0 0 0 0 1 11 123 1000 831 710 600 449 285 109 24 4 1 0 0 0 0 0 0 0 0 0 1 6 38 71 97 104 90 54 19 4 1 0 0 0 0 0 0 0 0 0 0 0 0 1 4 7 9 9 5 2 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 Original Plume Concentration 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 2 3 4 2 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 3 7 11 8 2 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 4 12 20 11 4 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 3 8 13 8 2 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 2 3 5 2 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 Plume after two years Extraction Only - No Added O2 Plume Concentrations 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3 2 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 2 7 6 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 6 15 10 3 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 2 8 7 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 2 5 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 9 8 3 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3 5 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 1 0 0 0 0 0 0 0 0 Plume after two years Plume after two years O2 Injected at 20 mg/L O2 Injected at 40 mg/L 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 Biodegradation Models • • • • • Bioscreen -GSI Biochlor - GSI BIOPLUME II and III - Bedient & Rifai RT3D - Clement MT3D MS • SEAM 3D Biodegradation Models Name Description Author 1 aerobic, microcolony, Monod Molz, et al. (1986) 1 aerobic, Monod Borden, et al. (1986) X 1 analytical f ir st-order Domenico (1987) BIOID 1 aerobic and anaerobic, Monod Srinivasan and Mercer (1988) X 1 cometabolic, Monod Semprini and McCarty (1991) X 1 aerobic, anaerobic, nutrient limitations, microcolony, Monod Widdowson, et al. (1988) X 1 aerobic, cometabolic, multiple substrates, f ermentative, Monod Celia, et al. (1989) BIOSCREEN 1 analytical f ir st-order, instantaneous Newell, et al. (1996) BIOCHLOR 1 analytical Aziz, et al. (1999) BIOPLUME II 2 aerobic, instantaneous Rifai, et al. (1988) X 2 Monod MacQuarrie, et al. (1990) X 2 denitrification Kinzelbach, et al. (1991) X 2 Monod, biofilm Odencrantz, et al. (1990) BIOPLUME III 2 aerobic and anaerobic Rifai, et al. (1997) RT3D 3 aerobic and anaerobic Clement (1998) X BIOPLUME Dimension Dehalogenation of PCE • PCE (perchloroethylene or tetrachloroethylene) Cl Cl Cl • TCE (trichloroethylene) Cl H • DCE (cis-, trans-, H C and Cl 1,1-dichloroethylene • VC (vinyl chloride) Cl C C PCE TCE C C H Cl Cl Cl H Cl C C C Cl DCE's C C H Cl H H C C Cl H Cl VC H H Dehalogenation • Dehalogenation refers to the process of stripping halogens (generally Chlorine) from an organic molecule • Dehalogenation is generally an anaerobic process, and is often referred to as reductive dechlorination R–Cl + 2e– + H+ ––> R–H + Cl– • Can occur via dehalorespiration or cometabolism • Some rare cases show cometabolic dechlorination in an aerobic environment Chlorinated Hydrocarbons • Multiple pathways • • • Electron donor – similar to hydrocarbons Electron acceptor – depends on human-added electron donor Cometabolic • Mechanisms hard to define • First-order decay often used due to uncertainties in mechanism Modeling Dechlorination • Few models specifically designed to simulate dechlorination • Some general models can accommodate dechlorination • Dechlorination is generally modeled as a firstorder biodegradation process • Often, the first dechlorination step results in a second compound that must also be dechlorinated Sequential Dechlorination • Models the series of dechlorination steps between a parent compound and a non-hazardous product • Each compound will have a unique decay constant • For example, the reductive dechlorination of PCE requires at least four constants • • • • PCE TCE DCE VC –k1–> –k2–> –k3–> –k4–> TCE DCE VC Ethene