AP Biology Notes
advertisement

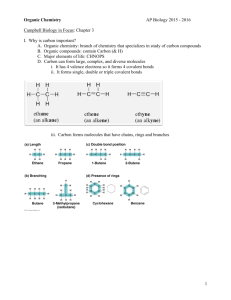
CARBON AND THE MOLECULAR DIVERSITY OF LIFE Chapter 4 Biology – Campbell • Reece IMPORTANCE OF CARBON Organic chemistry – the study of carbon compounds Most versatile building blocks of molecules It has 4 valence electrons Shares them in covalent bonds (not likely to form ionic) It makes large complex molecules possible. It determines an organic molecule’s three dimensional shape IMPORTANCE OF CARBON Variation in carbon skeletons contributes to the diversity of organic molecules Length Shape (straight, branched, ring) Number and location of double bonds Other elements covalently bonded to available sites HYDROCARBONS Contain only carbon and hydrogen Have a diversity of carbon skeletons which produce molecules of various lengths and shapes Are hydrophobic because the C-C and C-H bonds are nonpolar Biologically important molecules (fats) STRUCTURE OF HYDROCARBONS ISOMERS Compounds with the same molecular formula but with different structures and different properties Structural isomers = differ in the covalent arrangement of their atoms (and location of double bonds) Geometric isomers = share the same covalent partnerships, but differ in spatial arrangements. cis isomer – on the same side of the double bond trans isomer – on opposite sides ISOMERS Enantiomers = isomers that are mirror images of each other Can occur when four different atoms or groups of atoms are bonded to the same carbon. Two different spatial arrangements Usually, one form is biologically active and the other is not ISOMERS FUNCTIONAL GROUPS Contribute to the molecular diversity of life Have specific chemical and physical properties Are chemically active regions of organic molecules Behave consistently from one organic molecule to another Determine unique chemical properties of the molecules in which they occur HYDROXYL GROUP CARBONYL GROUP CARBOXYL GROUP AMINO GROUP SULFHYDRYL GROUP PHOSPHATE GROUP METHYL GROUP