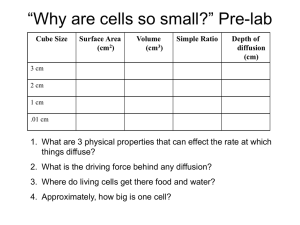
DATA TABLE: Diffusion In Agar Cubes
advertisement

Name:_______________________________________________Date:____ _____Period:______ Diffusion In Agar Cubes Is Bigger Better? OR Is Smaller Smarter? Adapted from Flinn Scientific Publication No. 10110 Introduction: Diffusion is one of the very important processes by which substances such as nutrients, water, oxygen, and cellular waste are transported between living cells and their environment. This activity will help you explore the relationship between diffusion and cell size by experimenting with model “cells.” In this experiment, you will use agar cubes to which the indicator phenolphthalein has been added. Phenolphthalein is an acid/base indicator that turns pink in the presence of a base such as NaOH. Thus the surface of the agar cubes will turn pink immediately when put into a NaOH solution. The NaOH will continue to diffuse through the cube and gradually turn the inside of the cube pink. The guiding question for this lab is thus: What determines the efficiency of diffusion throughout the model “cells”? Use this question to help formulate a hypothesis: Your hypothesis: Materials: Agar A ruler 0.1M NaOH per group 200mL Knife Large beaker Spoon/Tongs Procedure: 1. Each group will cut three agar cubes: A 3cm cube, a 2cm cube, and a 1cm cube. Also – cut a rectangular prism with dimensions of your choosing. CUT AS ACCURATELY AS POSSIBLE. 2. Pour 200mL of 0.1M sodium hydroxide solution into your 400mL beaker. 3. Immerse your 3 cubes and rectangular prism in the sodium hydroxide solution, noting the time. (WARNING: Sodium hydroxide is a strong base. If it spills, please contact teacher immediately.) 4. Let the cubes soak for approximately 8 minutes. 5. Periodically, gently stir the solution, or turn the cubes over. 6. After 8 minutes, use a spoon or tongs to remove the cubes from the sodium hydroxide solution. 7. Blot the agar with a paper towel. 8. Promptly cut each cube in half and measure the depth to which the pink color has penetrated. Sketch each block’s cross-section. (Use colored pencils.) 9. Record your measurements and sketch each cube (or rectangular prism) in your data table. 10. Do the following calculations for each cube (or rectangular prism) in your data table: Data: Create a data table in your lab notebook. Calculating % diffusion in each cube (note: your rectangular prism may differ): Calculate total volume of each cube (volume = L x W x H) Calculate volume that did not turn pink. (That is, calculate total volume of the small portion of the cube that did not turn pink – use the same formula L x W x H) Calculate volume diffused = total volume – volume not pink. Calculate % diffusion = Volume diffused /total volume x 100 Calculate the surface area of each cube and the surface area to volume ratio (note: your rectangular prism may differ): Calculate the surface area of a cube = L x W x # of sides Calculate surface area/volume ratio. Conclusion Questions: In the conclusion section of your lab notebook, answer the following questions. 1. In terms of maximizing diffusion, what was the most effective size cube that you tested? 2. Why was that size most effective at maximizing diffusion? What are the important factors that affect how materials diffuse into cells or tissues? 3. If a large surface area is helpful to cells, why do cells not grow to be very large? 4. You have three cubes, A, B, and C. They have surface to volume ratios of 3:1, 5:2, and 4:1 respectively. Which of these cubes is going to be the most effective at maximizing diffusion, how do you know this? 5. How does your body adapt surface area-to-volume ratios to help exchange gases? 6. Why can’t certain cells, like bacteria, get to be the size of a small fish? 7. What are the advantages of large organisms being multicellular? DATA TABLE: Cube Size Total cube volume (cm3) Diffusion In Agar Cubes Total volume that was not pink (cm3) Sketch of each Cube Volume of the diffused cube Percent Diffusion (total volume – volume that was not pink) Surface area of cube (cm2) Surface area to volume ratio 1cm 2cm 3cm Diffusion In Agar Cubes Name:___________ DATA TABLE: Cube Size 1cm 2cm 3cm Total cube volume (cm3) Total volume that was not pink (cm3) Sketch of each Cube Volume of the diffused cube (total volume – volume that was not pink) Percent Diffusion Surface area of cube (cm2) Surface area to volume ratio Preparation of agar 1. Mix 20g of agar with one liter of distilled or deionized water. Recipe based on 15 groups of students working in pairs. 2. Heat almost to a boil. Stir frequently until solution is clear 3. Remove from heat. AS the agar mixture cools add 10 mL of 1% phenolphthalein solution (1g phenolphthalein in 100 mL 95% ethyl alcohol) and stir. If mixture is pink, add a few drops of dilute hydrochloric acid until the pink color disappears. 4. Pour agar into a shallow tray to a depth of 3cm and allow it to set (over night). A tray measuring 12cm x 2cm that is at least 3cm deep will accommodate one liter of agar mixture.