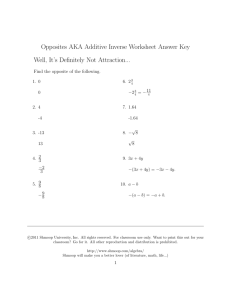
Biomolecules Discussion
advertisement
EQ: Distinguish between biomolecules by creating a 3d model of each and summarizing its structure and function in a graphic organizer. OBJ: Recognize structure and function of common biomolecules What is a Biomolecule? Organic molecule made by living organisms Consists mostly of carbon (C), hydrogen (H), and oxygen (O) But wait…What is an Organic Molecule? Organic Molecules: Contain carbon Considered the “chemicals of life” Inorganic Molecules: Do not contain carbon Monomers vs. Polymers Monomers: Molecules that may react with similar molecules to form a chain Polymers: A chain of many monomers that are chemically bonded together Animation of Condensation http://nhscience.lonestar.edu/biol/dehydrat/de hydrat.html Formation of Polymers How are polymers formed? Dehydration Synthesis (Condensation): Two hydrogen atoms and one oxygen atom are removed from the monomers to form water, and the two monomers are joined together. Animation showing Hydrolysis http://nhscience.lonestar.edu/biol/dehydrat/ dehydrat.html Breakdown of Polymers How are polymers broken down? Hydrolysis—the reverse of dehydration synthesis (condensation) Water added to the polymer, un-linking the chain and breaking it back down to its original monomer units Carbohydrates What are carbohydrates? Group of organic molecules that includes sugars, starches, and cellulose Carbohydrates Structure: Carbon, hydrogen, and oxygen in a 1:2:1 ratio (CH2O)n – n is an integer such as 5 (C5H10O5) Subunits: monosaccharides, such as glucose or fructose Most often in a ring shape Subunits are connected with covalent bonds. Monosaccharide Example Disaccharide Example Polysaccharide Example Carbohydrates Function: Energy Structural support Cell wall Cell membrane marker Lipids What are they? Organic molecule group including fats and phospholipids Lipids Structure: Subunits: ○ Glycerol and fatty acids ○ Glycerol and fatty acids plus phosphate group Insoluble in water Do not form large polymers (2 or 3 fatty acids with glycerol) ○ Examples: diglyceride and triglyceride Triglyceride Example Phospholipid Example Lipids Function: Energy storage Insulation Part of cell membrane (phospholipids) Hormones Proteins What are proteins? Group of organic molecules that provides structure and facilitates chemical reactions. Proteins Structure: Subunits: Amino acids Amino acids connect via peptide bonds Very large molecules Globular or structural Amino Acid Proteins Function: Lots of functions! Enzymes (speed rate of chemical reactions) Structural components in cells Mechanical functions in muscles and cytoskeleton (internal cell framework) Cell signaling Immune response Nucleic Acids What are nucleic acids? Group of organic molecules including DNA and RNA Nucleic Acids Structure: Subunits are nucleotides—5-Carbon sugar, Nitrogen base, and one or more Phosphate groups. Nucleic Acids Function: Storage and retrieval of information: ○ Encode genes ○ Gene expression Information Sources for Diagrams Capri, A. (2003). Carbohydrates. Retrieved from http://www.visionlearning.com/library/module_viewer.php?mid=61 Capri, A. (2003). Fats and proteins. Retrieved from http://www.visionlearning.com/library/module_viewer.php?mid=62 Indiana University. (2013). Fat and why it matters to you. Retrieved from http://www.indiana.edu/~oso/Fat/SolidNLiquid.html Cronk, J. (2012). Biochemistry dictionary. Retrieved from http://guweb2.gonzaga.edu/faculty/cronk/biochem/Hindex.cfm?definition=hydrogen_bond Chemical Education Digital Library Admin. (2011, January 20). Nucleic acid structure. Retrieved from Cronk, J. (2012). Biochemistry dictionary. Retrieved from http://guweb2.gonzaga.edu/faculty/cronk/biochem/Hindex.cfm?definition=hydrogen_bond Dna structure. (2010, September 28). Retrieved from http://www.pc.maricopa.edu/Biology/rcotter/BIO 205/LessonBuilders/Chapter 9 LB/Ch9b3.html Information Sources for this PPT Shmoop Editorial Team. (November 11, 2008).Biomolecules and the Chemistry of Life. Retrieved April 6, 2013, from http://www.shmoop.com/biomolecules/ Shmoop Editorial Team. (November 11, 2008).Organic vs. Inorganic Molecules Shmoop Biology. Retrieved April 6, 2013, from http://www.shmoop.com/biomolecules/organic-inorganic-molecules.html Shmoop Editorial Team. (November 11, 2008).Monomers, Polymers, and Dehydration Synthesis - Shmoop Biology. Retrieved April 6, 2013, from http://www.shmoop.com/biomolecules/monomers-polymers-dehydrationsynthesis.html Shmoop Editorial Team. (November 11, 2008).Lipids - Shmoop Biology. Retrieved April 6, 2013, from http://www.shmoop.com/biomolecules/lipids.html Shmoop Editorial Team. (November 11, 2008).Carbohydrates - Shmoop Biology. Retrieved April 6, 2013, from http://www.shmoop.com/biomolecules/carbohydrates.html Shmoop Editorial Team. (November 11, 2008).Proteins - Shmoop Biology. Retrieved April 6, 2013, from http://www.shmoop.com/biomolecules/proteins.html Shmoop Editorial Team. (November 11, 2008).Nucleic Acids - Shmoop Biology. Retrieved April 6, 2013, from http://www.shmoop.com/biomolecules/nucleicacids.html