Decay
advertisement

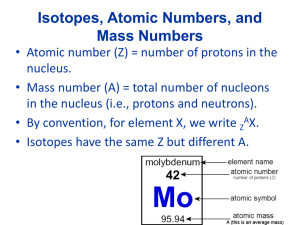
What is simplified in this picture? TheThompson: Discovery of the Electronthe Electron (Thomson) • Cathode Ray Tube • Charged particles produced (affected by magnetic field) • Concluded that atom must have positive and negative parts • Electron – negative part of the atom • Only knew the e/m ratio • Plum Pudding Model Charge and Mass of the Electron (Millikan) • Oil drop experiment • Determines charge on electron (uses electric field to counteract gravity) • Quantized • e = 1.602 X 10-19 C • m = 9.11 X 10-31 kg The Nucleus (Rutherford) • Gold Foil Experiment • Discovers nucleus (disproves Plum Pudding Model) • Planetary Model Atoms:Basic Facts Three particles Particle Charge Mass Proton +1 ~1 g/mol Electron -1 0.00055 g/mol (1/1837) Neutron 0 ~1 g/mol Photo of a single Barium atom Atoms:Basic Facts 1. Size – Measured in Angstroms 1 A = 1 X 10-10 m 1 Cl atom = 2.0 A 5 million Cl atoms can be lined up in 1 mm. Atoms:Basic Facts 1. Mass – grams/mole H = 1.00794 grams/1 mole 1.00794 g/6.022 X 1023 atoms C = 12 g/mol Isotopes 1. Isotopes – Atoms with the same # of protons, but different # of neutrons Copper-63 29 p 34 n Copper-65 29 p 36 n 2. Atomic Mass –weighted average of all the isotopes p n E 16 16 16 12 17 16 18 16 12 19 18 10 18 13C 90Sr 24Mg Ions 1. Cation – Positive Ion 2. Anion – Negative Ion 3. Review Common Charges P ClO2- Mg+ Mg2+ N E Stable vs. Unstable Nuclei Nuclear Changes 1. Most nuclei are stable – do not change 2. Some nuclei are unstable (radioactive) • • • Change into a different nucleus (decay) Spontaneous process – happens naturally, by itself Releases radiation Only nuclear reactions can change a nucleus. No chemical process can Radium Radon + Radiation Decay -New element and alpha, beta, or gamma -lost mass becomes kinetic energy Alpha particle Helium nucleus Types of Nuclear Radiation 4 ( 2He) Beta particle (0-1e) Gamma rays Positron (0+1e) 2 p+ 2n fast-moving electron. ehigh energy electromagnetic radiation positive electron (anti-matter) What Stops Radiation Paper Alpha () Beta () Gamma () Al Foil Lead. Wood Iron, Concrete Decay Equations Alpha Decay 238 U 4 He + 92 2 234 Th 90 Beta Decay 234 Th 0 e + 90 -1 234 Pa 91 Decay Equations Gamma Decay Occurs with alpha and beta decay No change in atomic mass (gamma radiation has no mass 00) Decay: Ex 1 What product is formed when radium-226 undergoes alpha decay? 226 Ra 88 4 He 2 + Decay: Ex 2 What element undergoes alpha decay to form lead-208? 4 2He + 208 82Pb Decay: Ex 3 What isotope is produced when thorium-231 beta decays? 90Th 231 0 -1e + Write the equation that describes oxygen-15 undergoing positron emission. Which nuclei are radioactive (unstable) 1. All elements have at least one radioactive isotope 2. All isotopes of elements heavier than Lead (82) 82 Pb At least one radioactive isotope 207.2 All isotopes are radioactive Transmutation • Rutherford(1919) – First successful alchemist 14 N + 4 He 17 O + 1 H 7 2 8 1 • Modern methods – Particle Accelerators (Cyclotrons) – Use neutrons or other elements (creation of transuranium elements) Periodic Table 1. 2. 3. 4. 5. Dmitri Mendeleev – 1869 Used atomic mass (modern is by atomic #) Period – Across Group – Down Metals Non-Metals Metalloids - Semiconductors Periodic Table Group 1 Alkali metals Group 2 Alkaline earth metals Transition Metals Group 7 Halogens Group 8 Noble Gases Lanthanides Actinides Discuss placement of Lanth/Act Average Atomic Mass 1. Atomic Mass – Weighted Average of all the isotopes Average Atomic Mass Calculate the ave atomic mass of Boron if it exists as 19.90% Boron-10 (10.013 g/mol) and 80.10% B-11 (11.009 g/mol)? (Ans: 10.811 g/mol) Mixing Elements Ionic = Metal + Non-metal (NaCl) Molecular = Non + Non (CH4) Alloy = Metal + Metal •Stainless steel (Fe/Cr) •Brass (Cu/Zn) •Bronze (Cu/Sn) Ionic vs. Molecular Ionic Compounds Start w/ Metal Stealing of Electrons Clumps of Ions(crystals) Called Salts No prefixes, may need Roman # Molecules Two Non-metals Sharing of electrons Separate Molecules Shapes (tetrahedral, etc…) Polar and Non-Polar Molecules (H2O vs CH4) Prefixes Ionic Solids Ionic vs. Molecular Ionic or molecular? HCl CO2 VO3 H2O BaF2 What ionic compound would form between: Ba and Cl Ba and Te Al and S Fe3+ and O Fe2+ and O H He Al Naming Ionics I. Binary Compounds Gr I and Gr II metals (and Aluminum) NaCl BaO Al2O3 magnesium bromide aluminum sulfide potassium oxide Naming Ionics I. Compounds with Polyatomics Sodium hydroxide Sodium carbonate Aluminum Sulfate NaNO3 Ca(OH)2 (NH4)3PO4 Mixed Examples Magnesium Sulfide Magnesium Sulfite Magnesium Sulfate Lithium Phosphide Lithium Phosphate Ba(ClO3)2 BaCl2 Roman # Ionics 1. Example Fe(II)and oxygen Fe(III) and oxygen How do we distinguish? 2. Metals which have multiple oxidation states Transition and post-transition metals Iron (III)Bromide Tin(II) nitrate Cobalt(III)Oxide CoCl2 MnO2 Ru2(SO4)3 VO3 Mixed Examples Calcium Bromide Chromium (III)Nitrate Aluminum Sulfate Iron(III)Carbonate Li2S CoCl2 Ti3N2 Mg(NO2)2 Magnesium Sulfide Magnesium Sulfite Magnesium Sulfate Mn2(SO4)3 Lithium Phosphide Lithium Phosphate Ba(ClO3)2 Copper(II)nitrate Household Ionics Many ionic compounds are called salts CaCl2 – Calcium Chloride (Quik-Joe) NaHCO3 – Sodium bicarbonate(Baking soda) CaCO3 – Calcium Carbonate(Chalk, antacid) NaOH - Sodium Hydroxide (Drano) MgSO4 – Magnesium Sulfate(Epsom Salts) All big clumps (crystals) of Ions Naming Molecules Prefixes mono hexa di hepta tri octa tetra nona penta deca May skip “mono” for first element CO CO2 Cl2O7 SF6 Chlorine dioxide Diphosphorus tetroxide Carbon tetrachloride Naming Molecules Household Molecules H2O HCl NH3 H2SO4 HC2H3O2 Review of all naming CS2 N2O NaI PCl5 FeF2 HgI2 K2CO3 NO3 Ba(OH)2 Calcium Chloride Silicon Dioxide Copper(II)Carbonate Magnesium Phosphate Dicarbon Octahydride Chromium(III)Oxide Dihydrogen Monoxide Acids 1. Binary Acids HCl HF H2S hydrochloric acid hydrofluoric acid hydrosulfuric acid Acids 2. Oxoacids HClO4 HClO3 HClO2 HClO Acid perchloric acid chloric acid chlorous acid hypochlorous acid Ion perchlorate (ClO4-) chlorate (ClO3-) chlorite (ClO2-) hypochlorite (ClO- ) Examples HCN HNO3 H2SO4 H2SO3 Examples HNO2 HI H3PO4 H3CO3 HClO3 H2 N2 O2 F2 Cl2 Br2 I2 Hydrogen Nitrogen Oxygen Fluorine Chlorine Bromine Iodine (H(N3(O2(F(Cl(Br(I- Hydride) Nitride) Oxide) Fluoride) Chloride) Bromide) Iodide) 26 1)Xe-130 has one more neutron 50) 1)Molecular PF5, SCl, N2O4 Ionic NaI Ca(NO3)2, FeCl3, LaP, CoCO3 32. 85.47 g/mol 56.a) CuBr2 b) Fe2O3 c) Hg2CO3 d) Ca3(AsO4)2 e) (NH4)2CO3 58. Na2O CaO FeO Al2O3 NaNO3 Ca(NO3)2 Fe(NO3)2 Al(NO3)3 Na2SO4 CaSO4 FeSO4 Al2(SO4)3 Na3AsO4 Ca3(AsO4)2 Fe3(AsO4)2 AlAsO4 64.a) Cu2+ b) Ag+ c) Al3+ d) Co2+ e) Pb2+ 1. 85.47 g/mol 2. Cu2+ Ag+ Al3+ Co2+ Pb2+ S2SO42ClO3OHCO32-