Energy for one molecule to escape
advertisement

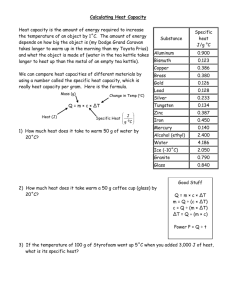
Energy for one molecule to escape 14 Matter very hot and cold To do: • You will be finding the energy required to release one water molecule from the liquid. When the energy is calculated on the basis of the energy per kilogram, the quantity is known as the specific latent heat of vaporisation. A preliminary check • The first step is to estimate the effective power output of the kettle. – Add enough water to the kettle to cover the element, then add about 100 g more. Measure the total mass of this water. – Measure the temperature of the water (or simply guess that it is around 15–20 °C). – Time how long it takes for the kettle to bring the water to a rolling boil. Calculation • Use the specific thermal capacity of the water (4200 J kg–1 K–1) to calculate how much energy must have been supplied to the kettle. Divide this energy by the time taken in seconds to estimate power supplied to the water. This value provides some correction for energy losses. Where might the energy go? The measurement • This part of the activity produces steam – be careful! – Place the kettle on the mass balance and switch it on. – Wait for the water to boil and then note the total mass of kettle plus water. Time how long it takes for about 50 g of water to boil away. Measure the actual mass loss as accurately as you can. Use your knowledge of the effective power output of the kettle and the time taken to calculate the energy supplied to boil off this mass of water. Now calculate the energy to liberate each molecule from the water – here are some steps to help you: • The molar mass of water is 18 g. How many moles of water did you boil off? • How many molecules of water were transferred from the liquid to the vapour? • How much energy was required to release each molecule into the vapour? • Make what comments you can about the reliability of your estimate. • Now compare this value with the average energy of the molecules at 100 °C. This is of the order kT, so the average energy is about 1.38 x 10–23 J K–1 x 373 K = 5.1 x 10–21 J How much water boiled off? m( water) 50 g m(molar ) 18 gmol 1 m( water) moles ( water) m(molar ) n 2.8 mol How much water boiled off? N NA n N 6 10 2.8 23 N 1.7 10 24 How much water boiled off? W P t W Pt W Pt Pt Energy / particle 1.7 10 24 You have shown that 1. The energy required to evaporate each molecule is roughly 7 x 10–20 J. 2. The energy required to evaporate one molecule at 100 °C is about 15 times the average energy of the molecules (15 kT) at that temperature.