Matter, Gas Behavior, Changes of State, and Atoms Study Guide
advertisement

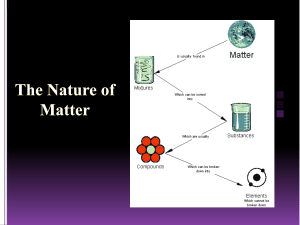
Name: ____________________________ Date: _________ Period: ________ Bin# ___ Matter, Gas Behavior, Changes of State, and Atoms Study Guide ANSWER KEY 1. What was J.J. Thomson’s atomic theory? That atoms contain negatively charged particles called electrons. 2. What was the main idea of Dalton’s atomic theory? That all atoms of the same element are exactly alike and have the same mass. 3. Define the law of conservation of mass. The law of conservation of mass states that matter cannot be created or destroyed, matter can only change form. 4. Where is most of the mass of an atom found? Most of an atom’s mass is found in the nucleus. 5. What are the charges of a a. Proton – Positive b. Neutron – No charge c. Electron – Negative 6. What is a chemical property? A chemical property is the characteristics that describe the ability of an object to change into a new substance. 7. Give an example of a chemical property. Flammability of a gas, reactivity with water 8. What is a physical property? A physical property is a characteristic of an object that can be observed. 9. Give an example of a physical property. Color, shape, size, state, conductivity, malleability, solubility, etc. 10. What is a physical change? Any change that alters the form or appearance of matter but does not make any substance in the matter into a different substance 11. Give an example of a physical change. Boiling water, melting ice, cutting the grass. 12. What is a mixture? Made of two or more substances where each substance keeps its individual properties. 13. What is a homogeneous mixture? A mixture in which substances are evenly distributed throughout the mixture. 14. Give an example of a homogeneous mixture. Salt water, coffee, milk 15. What is a heterogeneous mixture? A mixture in which the different parts of the mixture can be seen. 16. Give an example of a homogeneous mixture. Salad, chocolate chip ice cream, vegetable soup, granite. 17. What is chemistry the study of? The properties of matter and how matter changes. 18. Explain what an atom is? An atom is the basic unit of all living and non-living matter, the smallest particle of an element t that has all the properties of that element. 19. What happens to a gas when the temperature of a gas increases at a constant pressure? The pressure will increase when the temperature increases. 20. What is Charles’s law? When the temperature of a gas increases at a constant pressure, its volume increases. When the temperature of a gas decreases at a constant pressure, its volume decreases. 21. Give an example of the Charles’s law. A balloon being dipped into liquid nitrogen, shrinking, then removed from the liquid nitrogen, and returning back to its original size. 22. What is Boyle’s law? Boyle’s law states that when the pressure of a gas increases, the volume of the gas decreases. Name: ____________________________ Date: _________ Period: ________ Bin# ___ 23. Give an example of Boyle’s law. When a plunger is being pushed into an air pump, the space within the air pump gets smaller (the volume decreases). As the volume decreases, the pressure increases and forces air into the tires. 24. How are the particles arranged in a a. Liquid? Free to move about within a container but remains in contact with one another. b. Solid? In a fixed position, moves by vibration and all of the particles are very close to each other. c. Gas? Free to move about within a container. Particles have so much energy they overcome moving in contact with one another. 25. A solid is a state a of matter with Definite volume and a Definite shape. 26. What is matter? Anything that has mass and takes up space. 27. How would you measure the volume of a regular object? Measure the length, width and height and multiply each by each other. L X W X H 28. How would you measure the volume of an irregular object? You would measure an irregular object’s volume by placing it into a graduated cylinder and measuring its displacement. 29. What are elements composed of? All elements are composed of extremely small particles called atoms. 30. How do you calculate the density of an object? Density = Mass / Volume 31. The density of a block of wood with a volume of 160cm3 and a mass of 40 grams is 0.25g/cm3 32. How do you express the unit for density of a solid object? g/cm3 33. How do you express the density of a liquid? g/mL 34. What is mass? Mass is the amount of matter that an object is made up of. 35. What is an element? An element is a substance that CANNOT be broken down chemically into another substance.