VSEPR Molecular Shapes 2013
advertisement

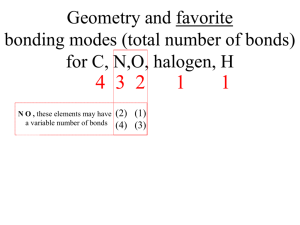
1. Do the warm-up on the table and check your answers (Polyatomic and Acids) 2. Get the home work off of the front table 3. Draw the Lewis Structures for the molecules on the board on a PIECE OF PAPER (not white boards) 4. Have periodic table, paper, and pencil ready for lesson 5. Today we will be answering: “What does the Lewis Structure tell us about the Covalent molecule” Homework: Naming Worksheet Memorizing Polyatomics Important Dates: 2/12 Polyatomic quiz 2/24 Covalent Test FIVE E- GEOMETRY GROUPS: 2 electron groups – Linear 3 electron groups – Trigonal Planar 4 electron groups – Tetrahedral 5 electron groups – Trigonal bipyramidal 6 electron groups – Octahedral Example: CO2 Two e- Groups around central atom Shape Name: Linear Angles 180° sp hybridization Three e- groups around central atom Two types of Trigonal Planar groups: 3 bonding pairs 2 bonding pairs and 1 lone pair Example: BH3 Three bonding electron pairs Shape Name: Trigonal Planar Angles 120° sp2 hybridization Examples: SO2 Two bonding electron pairs, one lone pair Shape Name: Bent Angles less than 120° sp2 hybridization Four e- groups around central atom Three types of tetrahedral groups: Four bonding pairs Three bonding pairs, one lone pair Two bonding pairs, two lone pairs Example: CCl4 Four bonding pairs around central atom Shape Name: Tetrahedral Angles: 109.5° sp3 hybridization Example: PF3 Three bonding pairs, one lone pair Shape Name: Trigonal Pyramidal Angles less than 109.5° sp3 hybridization Example: H2O Two bonding pairs, Two lone pairs Shape Name: Bent Angles less than 109.5° sp3 hybridization Five e- groups around central atom Four types of Trigonal Bipyramidal groups: Five bonding pairs Four bonding pairs, one lone pair Thee bonding pairs, two lone pairs Two bonding pairs, three lone pairs Example: PF5 Five bonding pairs Shape Name: Trigonal Bipyramidal Angles 90° and 120° sp3d hybridization Example: SF4 Four bonding pairs, one lone pair Shape Name: Seesaw Angles less than 90° and less than 120° sp3d hybridization Example: BrF3 Thee bonding pairs, two lone pairs Shape Name: T-Shaped Angles less than 90° sp3d hybridization Example: XeF2 Two Bonding pairs, three lone pairs Shape Name: Linear Angles 180° sp3d hybridization Six e- groups around central atom Three types of Trigonal Bipyramidal groups: Six bonding pairs Five bonding pairs, one lone pair Four bonding pairs, two lone pairs Example: SF6 Six bonding pairs Shape Name: Octahedral Angles 90° sp3d2 hybridization Example:BrF5 Five Bonding Pairs, One lone pair Shape Name: Square Pyramidal Angles less than 90° sp3d2 hybridization Example: XeF4 Four bonding pairs, two lone pairs Shape Name: Square Planar Angles 90° sp3d2 hybridization