Carbohydrate Lab
advertisement

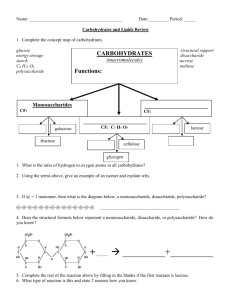
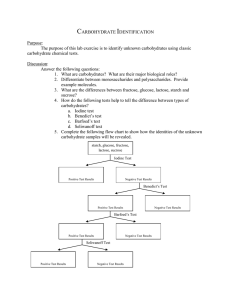
Carbohydrate Lab Name: Read the following and answer the pre-lab questions before conducting the experiments. Introduction: Carbohydrates are an important energy source for all living organisms and are used in making cell structures. The three types of carbohydrates are monosaccharides, disaccharides, and polysaccharides. Saccharide means sugar. All carbohydrates contain carbon, hydrogen and oxygen in a 1:2:1 ratio. Figure 1 illustrates the structural formulas for three different monosaccharides. These are the monomer units of all carbohydrates. Disaccharides form when two monosaccharides react together in a dehydration synthesis reaction. Water is also a product of this reaction. A polysaccharide is formed after many dehydration synthesis reactions have taken place. Conversely, a polysaccharide (polymer) can split into its monomer units, the monosaccharides, during a hydrolysis reaction in which water is needed as a reactant. Chemical tests can be used to determine if an unknown carbohydrate is a mono-, di-, or polysaccharide. The Benedict’s solution test, which is a blue solution, will identify monosaccharides with a color change to red or orange. The iodine solution test, which is a rusty orange colored solution, will identify polysaccharides with a color change to a deep blue-black or purple color. If, after doing both tests and neither results in a color change then the unknown sugar would be a disaccharide. The experimenter will use these tests to determine the type of carbohydrate for several common food items such as honey, apple juice, milk sugar, table sugar, oats and corn starch. Fig. 1 Glucose Fructose Materials test tubes labeling tape hot plate honey table sugar oats test tube rack pencil 250 ml beaker apple juice milk sugar iodine solution test tube holder droppers monosaccharide solution disaccharide solution corn starch Benedict’s solution Procedures: 1. Complete all prelab questions after having read the entire packet. 2. Check off each procedure with your pencil as you complete it. 3. The Benedict’s Test for Monosaccharides: a. Fill a 600 ml beaker half full of water. b. Bring water to boil on hot plate. This is called a hot water bath. c. While waiting for water to boil, label 3 test tubes (M, D, and P) with labeling tape. M, D, and P stand for mono-, di- and polysaccharide. These are our “known”. carbohydrates. We will use them as a comparison for determine type of carbohydrate (mono-, di-, polysaccharide) for the unknown sugars in procedure 5. d. Using the 10ml graduated cylinder, obtain 4 ml of the monosaccharide sugar from the prep table. (If you get extra DO NOT pour back into STOCK FLASK). Pour extra down the drain or give it to another group. e. With the 4 ml, pour approximately 1ml into a well of the plastic reaction plate (the well should be approximatly ½ filled. With the rest of the sugar, pour into the test tube labeled M. f. Follow procedures 3d and 3e for the di- and polysaccharides. g. Once all 3 test tubes are prepared, add 20 drops of Benedict’s solution to each. h. Using the test tube holders, place Tubes M ,D, and P into the hot water bath. i. Wait approximately 3-5 minutes to see if there are any color changes. A change from the original blue of the Benedict’s solution to a red or orange color indicates that a monosaccharide is present. The original blue color will remain if a disaccharide or polysaccharide is present. j. Record these color changes in Table 6.1 4. The Iodine Test for Polysaccharides: a. Using the Mono-, Di, and Polysaccharides that have been placed in the reaction plate wells. b. Add 2 to 3 drops of iodine solution to each well. c. Mix the contents with a glass rod. d. Observe the color changes that occur after mixing. A change from the original iodine or rusty orange color to a deep blue-black or purple indicates a polysaccharide is present. The original orange color will remain if a mono- or disaccharide is present. e. Record these color changes in Table 6.1 5. Unknown carbohydrate classification using Benedicts and Iodine. a. In the first column of Table 2, labeled “Carbohydrate” write the names of the food items to be tested. Then, using 6 test tubes and 6 wells, repeat procedures 3 and 4. Pre-lab Questions: Must be completing before beginning lab. 1. Name the three elements found in all carbohydrates. 2. What does “saccharide” mean? 3. Give three examples of monosaccharides. 4. For the three monsaccharides that you listed above, count the total number of carbons, hydrogens, and oxygens in the illustration. Give molecular formulas for each. 5. Closely examine the structural formulas of the three monosaccharides. a. What do they all have in common? b. How are they different? 6. Benedict’s solution tests for which type of carbohydrate (mono-, di- or polysaccharide? 7. How will you know if you have a monosaccharide after you add Benedicts to your substance? 8. What will your substance look like after adding Benedict’s if you have a disaccharide or a polysaccharide? 9. How much of each sugar solution will you use for the Benedict’s and Iodine test? 10. How much Benedict’s solution will you add to each sugar? 11. How much Iodine will you add to each sugar? 12. After adding the Iodine solution, how will you know if you have a polysaccharide? 13. After adding the Iodine, how will you know if you have a mono- or disaccharide? 14. Name four of the possible unknown carbohydrates that you will be testing to see if they are mono-, di- or polysaccharides. 15. Why should you use a clean dropper for each solution? Table 1 – Results of Tests with Known Carbohydrates Tube Number 1 2 3 Carbohydrate Type Benedict’s Color After Heating Iodine Color Monosaccharide Disaccharide Polysaccharide Table 2 – Results of Tests with Unknown Carbohydrates Food Item Benedict’s Color Iodine Color Type of Carbohydrate Analysis Questions: Use your data tables to answer question 1-6. 1. Name the three categories of carbohydrates studied in this investigation. 2. What three elements are present in all carbohydrates? 3. How many times larger is the number of hydrogen atoms than oxygen atoms in all carbohydrates? 4. “Mono-“ means one, “di” means two, and ”poly-“ means many. Why are these terms used in describing the three types of sugars? 5. How can you tell by using Benedict’s and Iodine solutions if a sugar is a: a. monosaccharide? b. disaccharide? c. polysaccharide? 6. A certain sugar has a color change in Benedict’s solution. a. Can you tell what type of saccharide it is? b. Explain your answer. c. Give example of food items not used in this lab that are: a. monosaccharide b. disaccharide c. polysaccharide