CDCP - 02.28.11 - Titration Excel Practice
advertisement

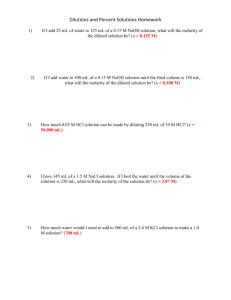
Science 10 Brakke CDCP – Titration Example for Excel Practice ECA – Chemistry Topic 05 It is apparent that many of you need direction in sorting, processing, and creating data tables within excel and organizing them into a lab report in word. The following explanation is the results of a titration lab – this is not the proper way to report data from a lab. Your task is to take the following data organize it in a data table so that the reader can more easily view, critique, and compare your results with theirs. The following data was collected during the titration of Vinegar, also known as Acetic Acid (CH3COOH, MW = 60.05g/mol). Before the titration of vinegar with NaOH, the molarity of the NaOH needed to be determined ahead of time so the solution was standardized using a known concentration of potassium hydrogen phthalate, also known as KHP (KHC8H4O4, MW = 204.23 g/mol). To standardize the NaOH, it was titrated with three times with samples of KHP with a mass of (trial 1 = 1.165g, trial 2 = 1.276g, trial 3 = 1.345g) each being dissolved in 100.0mL of deionized water. When titrated, trail 1 had a starting volume of 0.23mL and an ending volume of 23.08mL, trial 2 had a starting volume of 0.56mL and an ending volume of 24.01mL, and trial 3 had a starting volume of 0.05mL and an ending volume of 26.41mL, and these values were used to calculate the total volume of NaOH used for the standard titrations. The molarity of NaOH was then calculated. The calculated concentration was then used to titrate solutions of vinegar in five trials. For each of the five trials, ~10mL of vinegar was precisely measured and then diluted to a volume of about 50mL (not precise as it does not change the total number of moles in vinegar). Each of the five solutions were made and the volume of vinegar was 10.05mL, 12.25mL, 9.56mL, 9.95mL, and 10.00mL for trials 1, 2, 3, 4, and 5 respectively. Each titration was then completed individually. For trial 1: 0.35mL and 33.95mL for the starting and ending volumes of NaOH respectively. For trial 2: 0.08mL and 43.69mL for the starting and ending volumes of NaOH respectively. For trial 3: 0.25mL and 29.89mL for the starting and ending volumes of NaOH respectively. For trial 4: 0.01mL and 32.65mL for the starting and ending volumes of NaOH respectively. For trial 5: 5.70mL and 40.10mL for the starting and ending volumes of NaOH respectively. The molarity was calculated for each trial, and averaged. The percent by mass of Acetic Acid in vinegar was then calculated using the average molarity (mol/L) and converting to total grams in 1 Liter using the molar mass of vinegar (giving units of g/L of water) and the % by mass was calculated using the known density of water (1g/mL or 100g/L). I was then provided that the precise concentration of NaOH was 0.30M, the concentration of vinegar was 0.83 g/mol, and the % by mass of acetic acid in vinegar is 5.0%. A percent error was calculated for each of these values. Using excel, provide a data table for the entire lab. How many Tables do you need? (I can think of 5) You need to include, raw data, processed data, etc This lab does not lend well to graphs, but we can make one, a graph of the final titration would have what units? (think of the variables) Copy and paste these tables and graphs into a word document and make any adjustments needed to the format. Make another table within word that provides any and all equations used along with examples o Volume of solution o g mol conversion o Molarity o Titration o Average o % Acetic Acid in Vinegar o % Error Write a conclusion for the lab (be sure to state your major results to start with). This will include major results, error analysis, etc. Since we did not do the lab, you are not required to reflect on the evaluation and improvement of the lab. When using excel: Use a small font that’s easy to read – such as Calibri 9 Every table should have a title Each column and row should have a description with units (grams(g), volume of base(mL), etc) If excel cuts off your significant figures (ex 21.00g 21g) you can highlight data and right click/format cells/number/decimal places Excel will complete mathematical calculations for you, try it out! You do not need a calculator for this data processing! The last and final piece would be uncertainty, we will get there soon enough