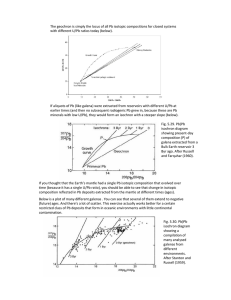
Geological and
advertisement

Foundations of a modern approach to measuring
geological age
~1900: Becquerel & Curie discover radioactivity in U, Pu, Ra and ‘ionium’ (Th)
Rutherford proposes 3 types of radioactivity:
emits mass but no charge (4He nucleus)
emits charge but no (observable) mass (electron or positron)
emission has neither charge nor mass (high-frequency radiation)
Rutherford notes/postulates two key properties of radioactivity:
• Reactions are exothermic
• Emission is independent of properties or environment of elements
If rate of emission is invariant w/ time or setting, then
radiation can serve as a clock:
Constant of proportionality;
now called ‘decay constant’
- dN/dt = N
1/ = ‘mean life
ln2/ = ‘half life’
(a miracle of integration occurs)
N = N0e-t
For and radiation, nothing lasting is produced (at least, nothing detectable
by 1900-era scientists). But particles accumulate in a measurable way:
Define ‘D’ as number of ‘daughter’ particles
D = D0 + D*
D* = N0 - N
D = N0(1-e-t) + D0 = N (et-1) + D0
Re-arrange decay equation to make time the dependant variable:
Pick mineral with no structural He; D0 = 0
t=
0) ] +1}
ln {[ (D-D
N
Radiation counting in lab
Pick mineral w/ stoichiometric
Parent element (e.g., UO2), so
N depends only on mass
With correct choice of sample, t depends only on D - the amount
of He trapped in the mineral lattice
Rutherford’s chronometer
U ~ 1.5x10-10
U
Pitchblende, or U ore, rich in UO2
8
1 gram of UO2
Time (yrs)
moles He
1000
5x10-9
1 million
5x10-6
10 million
5x10-5
1 billion
5x10-3
cc STP
1x10-4
0.1
1.0
100
Found African pitchblende is ca.
500 million years old
Problems:
• Sensitivity and precision of manometric measurements
• Reaction is not fully described. U weighs ca. 238 g/mol;
8 He nuclei only 32 g/mol. Where is the rest of the mass!
• He is not well retained by crystals
Breakthrough: Aston’s positive ray device
Ions are passed through a magnetic field oriented orthogonal
To their direction of motion. Ions are deflected with a radius
of curvature set by the force balance between the magnetic field
(qv x B) and the centripital force (mv2/r). That is, r = mv/(qB)
Low momentum
(low mass))
High momentum
(high mass)
If energy is of all ions is equal, this acts as a mass filter.
Strength of B field
Intensity
Finnigan Triton
A modern thermal ionization mass spectrometer
Momentum analyzer (electro magnet)
Ion source
Collectors (faraday cups
and/or electron multipliers)
Advances stemming from mass spectrometry
• Precision improves from ca. ±1 % to ca. ±10-5
• Recognition of isotopes permits the definition of decay reactions
Zprotons + Nneutrons = Amass
decay: Z + N
(Z-2) + (N-2) + 4He + + Q
e.g., 238U
234Th
147Sm
decay: Z + N
143Nd
+ 4He; = 6.5x10-12 yr-1
(Z+1) + (N-1) + e- + + Q
e.g., 87Rb
e.g., 14C
decay: Z + N
+ 4He; = 1.55x10-10
87Sr
14N
+ e-; = 1.42x10-11 yr-1
+ e-; = 1.2x10-4 yr-1
(Z-1) + (N+1) + e+ + + Q
e.g., 18F
18O
+ e+; = 3.3x103 yr-1
Most geological ‘chronometers’ depend on and decay
Mass spectrometry is best at measuring relative abundances of
isotopes. This motivates an additional change to age-dating equations:
D = Daughter (4He; 87Sr; 143Nd)
N = Parent (238U; 87Rb; 147Sm)
S = Stable (3He; 86Sr; 144Nd)
The ‘stable’ nuclide is always a non-radioactive, non-radiogeneic
isotope of the same element as the ‘Daughter’ nuclide.
D = N (et - 1) + D0
D/S = N/S (et - 1) + D0/S
Y-axis value
Slope
Y-intercept
X-axis value
This is the equation for a line in the ‘isochron’ plot
The anatomy of the isochron diagram
Measured composition
of object
D/S
m = et - 1
D0/S
N/S
Three strategies for use:
• Measured objects known to have D0/S ~ 0
• Assume or infer D0/S from independent constraint
• Define slope from two or more related objects, yielding
both age (t) and D0/S as dependent variables. These objects
must be of same age, have started life with identical D0/S,
but differ significantly in N/S
A common example:
the Rb-Sr chronometer applied
to granite
Isotopes of Sr:
84Sr:
0.56 %
86Sr: 9.87 %
87Sr: 7.04 %
88Sr: 82.53 %
(all values approximate)
Sr: typically a +2 cation; 1.13 Å ionic radius (like Ca: +2, 0.99 Å)
Isotopes of Rb:
85Rb:
Stable
87Rb: Radioactive: l = 1.42x10-11 yr-1;- decay
85Rb/87Rb
in all substances from earth and moon assumed = 2.59265
Rb: typically a +1 cation; 1.48 Å ionic radius (like K; +1, 1.33 Å)
The Sm-Nd chronometer
Isotopes of Nd:
Isotopes of Sm:
142Nd:
144Sm:
27.1 %
143Nd: 12.2 %
144Nd: 23.9 %
145Nd: 8.3 %
146Nd: 17.2 %
(147Nd: 10.99 d half life)
148Nd: 5.7 %
150Nd 5.6 %
(all values approximate)
3.1 %
(146Sm: 108 yr half life)
147Sm: 15.0 % (1.06x1011 yr half life)
148Sm: 11.2 %
149Sm: 13.8 %
150Sm: 7.4 %
(151Sm: 93 year half life)
152Sm 26.7 %
154Sm: 22.8 %
(all values approximate)
Normalized abundance
The ‘rare earth’ elements
Plagioclase
Garnet
Pyroxene
A fragment of the chondritic meteorite, Allende
A thin section of the chondritic meteorite, Allende
Comparison with a modern ‘Kelvinistic’ argument:
Summary of typical stellar lifetimes, sizes and luminosities
"There is one independent check on the age of the solar system determined by radioactivity in meteorites.
Detailed theoretical studies of the structure of the sun, using its known mass and reasonable assumptions
about its composition, indicates that it has taken the sun about five billion years to attain its present observed
radius and luminosity.”
W. Fowler
14C
14C
decay: The basis of most ages for geologically young things
is produced in the atmosphere:
14N
+ n = 14C + p
Cosmic-ray fast neutrons
Undergoes beta-decay with a half-life of 5730 yrs:
14C
= 14N + e-
= 1.209x10-4 yr-1
Age (yrs) = 19,035 x log (C/C0)
[ or …’x log (Activity/Activity0)’]
Key for application is assumption of a value of C0, which depends on
14C/12C ratio in atmosphere
Real applications require correction for natural isotopic fractionation
(e.g., during photosynthesis) and must consider variations in production
rate with time and isotopic heterogeneity of surface carbon pools
The ‘bomb spike’
Natural heterogeneity: 14C ‘ages’ of deep ocean water
Variation in atmospheric 14C/12C
through time due to natural processes
∆14C = (Ri/R0 -1)x1000
Where Ri = 14C/12C at time of interest
R0 = 14C/12C of pre-1890 wood
projected forward to 1950 (?!?&*!)
Using 14C to reconstruct earthquake
recurrence intervals
The U-Pb system and the age of the Earth
= 1.55125x10-10 (4.5 Ga half life)
= 9.8485x10-10 (0.7 Ga half life)
238U
= 206Pb + 8x4He
235U = 207Pb + 7x4He
204Pb
is a stable isotope
238U/235U is (nearly) constant in nature = 137.88
206Pb
204Pb
207Pb
204Pb
207Pb
=
=
-
206Pb
0
204Pb
207Pb
0
204Pb
204Pb
206Pb
206Pb
0
-
+
+
204Pb
235U
(et - 1)
(et - 1)
204Pb
207Pb
0
204Pb
204Pb
238U
204Pb
1
=
(et - 1)
137.88 (et - 1)
207Pb
-
204Pb
206Pb
204Pb
207Pb
0
204Pb
-
206Pb
0
204Pb
QuickTime™ and a
TIFF (LZW) decompressor
are needed to see this picture.