Unit 1 Everything
advertisement

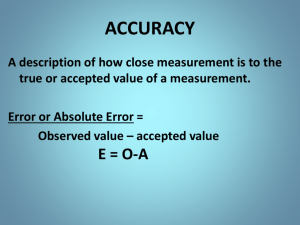
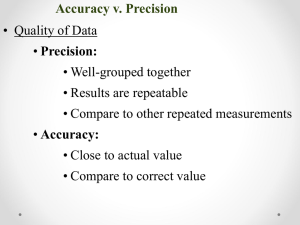
Fundamentals of Chemistry Unit One Scientific Method and Measurement Scientific Method Scientific Method: A way to Solve Problems. Steps of the Scientific Method Step 1: Identify the problem To begin the scientific process, a problem must be clearly and specifically identified. Step 2: Gather Information Before setting out to find the answer to a scientific question, information must be gathered in the form of preliminary research. Sources such as journals and scientific papers could be checked for existing information on the problem at hand. Step 3: For m a Hypothesis Once a problem or question has been recognized, a hypothesis or educated guess is constructed. In an experiment, hypotheses are not “correct” or “incorrect”, they are supported or not supported by the data collected. if a hypothesis has be tested repeatedly and not disproven it is known as a theory or postulate. Theory answers the question “Why?” (a theory explains what happened) A rule of nature is known as a law. Ex. Law of attraction and repulsion, Law of universal gravitation. Lawanswers the question “what?” (Law tells what happens) Step 4: Experimentation and Observation – Used to test your hypothesis. Data collected during this step needs to be organized and analyzed. -2 types of data: Qualitative & Quantitative. ▪Qualitative made using the five senses ▪Quantitative made using instruments **Think about qualitative as “what” and quantitative as “how much” A controlled experiment contains only 1 experimental variable. -Variable any factor affecting the outcome of an experiment. ▪Independent variable is set and controlled by the experimenter (such as time) ▪Dependent variable changes based on what is done to the experimental variable. (such as plant growth) Properly set-up experiments provide a control group as well as an experimental group. -Control groups are set-up under “normal” conditions and are used to compare to the experimental groups. Step 5: Draw Conclusions interpretations of experimental results. reference should be made to the original hypothesis. Questions to think about in the conclusion may include: *Was the hypothesis supported or not supported by the data? *What were possible sources of error in the lab? *What are some ways to improve the experiment? *What are some questions yet to be addressed? Important considerations: 1) If the data does not support the initial hypothesis, the experiment was not pointless! 2) If the original hypothesis or problem addressed needs to be modified, the whole process needs to start over. Sometimes an experiment that “goes wrong” opens the doorway to a new discovery. How Much Liquid is in Each Graduated Cylinder? Significant Figures: a system for representing measured values with the correct degree of accuracy. Its main purpose is to know how much to round off answers calculated from any measurement. A "significant" figure is a figure that is considered accurate. Significant figures: all of the digits that are known in a measurement plus a last digit that is estimated. Measurements and calculations should ALWAYS be recorded to the correct number of significant figures! Example: Room temperature 25.4ºC Suppose you were given temperature data for various points in Frederick from a variety of sources and the data looked like this: 23.232ºC, 25.2ºC , 26.1746ºC , 27.12ºC. When you calculate the average, how many sig figs should keep? 3 significant figures (25.4ºC) General Rule of Sig Figs An answer cannot be more precise than the least precise measurement from which it was calculated. RULES to identifying the number of sig. figs.: 1)Every Nonzero digit in a reported measurement is significant • 56.6, 2.34 and 978 all have 3 sig. figs. 2)Zeroes between nonzeroes are significant. • 6007, 50.89 and 5.708 all have 4 sig. figs. 3)Leading zeros appearing in front of nonzero digits are placeholders and not considered significant. • 0.000091, 0.042 and 0.42 all have 2 sig.figs. 4) Zeros at the end & to the right of a decimal point are ALWAYS significant. • 57.00, 2.030 and 7.000 all have 4 sig. figs. 5) Zeros at the end & to the right without a decimal point are NOT significant unless a careful measurement was actually made (which will have a decimal point after - 10.) • 400, 4000 and 30000 all have 1 sig. fig. When calculating the correct number of sig figs in an answer, perform all of the calculations first then round the final answer. Summary 1. If you are not a zero, you are significant. 2. If you are a sandwiched zero, you are significant. 3. If you are a zero at the end of a number, and there’s a decimal in your number, you are significant. 4. If you are a zero at the end of a number, and there’s NOT a decimal in your number, then you are NOT significant. 5. If you are a zero and you are at the beginning of a number, you are NOT significant. Adding or subtracting round to the same number of decimal places as the measurement with the least number of decimal places. Examples 3.451 + 1.41 + 2.072 = 6.933 6.93 (2 dec.) 7.982 + 1.02 + 2.1 = 11.102 11.1 (1 dec. ) 3.45 – 1.1 = 2.35 2.4 (1 dec.) Multiplying or dividing round the answer to the same number of significant figures as the measurement with the least number of significant figures. examples: 2.1 x 1.301 = 2.7321 2.7 (2 sig figs) 4.02 x 2.945 = 11.8389 11.8 (3 sig figs) 0.034 x 3.223 = 0.109582 0.11 (2 sig figs) Scientific Notation Review Scientific Notation A number written in scientific notation has two components: -a coefficient and 10 raised to a power •Coeffiecient must be greater than or equal to 1 and less than 10. Examples: 2.3 x 103 7.9 x 10-5 What is 540,000 in scientific notation? -Number between 1 & 10 = 5.4 -Move the decimal 5 times 5.4 x 105 5.4 x 10 = 54 54 x 10 = 540 540 x 10 = 5,400 5,400 x 10 = 54,000 54,000 x 10 = 540,000 5.4 x 105 = 540,000 Power can be positive or negative positive power of ten, the decimal moves one place to the right. Examples: 4.67 x 103 = 4670 2.71 x 104 = 27100 negative power of ten, the decimal point moves one place to the left. Examples: 4.5 x 10-6 = 0.0000045 1.21 x 10-3 = 0.00121 *Remember: 10-1 = (1/10) = 0.1 3.2 x 10-1 = (3.2/10) = 0.32 Accuracy & Precision Counting is exact!! Ex. 26 students in this class Counted numbers are considered to have infinite precision. Measurements are subject to error. (Errors reflect limitations in the methods used to make the measurement.) -Examples of error: incorrectly calibrated equipment or uncertainty of equipment or uncontrollable human error If each of the individual measurements in a set of data are close to the average of the set, then they are precise. Precision Precision of an individual measurement is determined by the markings on the piece of equipment used to take the measurement. REMEMBER: the number of significant figures in a measurement is determined by: -all of the known digits in the measurement (indicated by the markings on equipment) -plus one digit that is estimated (how far in between the markings). Precision Example A thermometer marked in whole ºC and you read it as 25.5ºC . -you would be estimating 1 decimal place!! -the 25 degrees in known and the .5 is estimated. (It was marked to the whole degree, so you can only estimate to the tenth.) **Only estimate 1 place beyond what you can read for sure!!** Accuracy Accuracy refers to the closeness of the average of the set to the “accepted” value and depends on how carefully the measurement was made. Example: A block of wood has a length of 4.3cm. -Student 1 measures the length of the block and finds it to be 4.4cm. -Student 2 measures the block of wood and determines its length to be 5.2 cm. *Which measurement is more accurate? 4.4cm Metal Ruler: Object is more than 12.3cm and just less than 12.4cm. The smallest marking represents 0.1cm, so you estimate to the nearest 0.01cm. The length of the object would be accurately and precisely recorded as 12.39cm. The last digit is estimated, so, 12.31 – 12.39cm would be acceptable. Plastic Ruler: Object is more than 12cm and less than 12.5cm. The smallest marking represents 0.5cm, so you estimate to the nearest 0.1cm. The length of the object would be accurately and precisely recorded as 12.4cm. The last digit is estimated, so, 12.1 –12.4 cm would be acceptable. (Up to 0.5 cm off) Which ruler is more precise? From 200.51g to 200.57g How much is the smallest marking worth? 0.1 gram (you can read it to the tenth’s place!!) What are the digits of this mass you know for certain? 200.5 grams(hundreds, tens, ones, tenths marked) Next you always estimate 1 place beyond. 200.53 grams Good or Poor?? Good accuracy Poor accuracy Good precision Good precision Good or Poor?? Good accuracy (on average) Poor precision Poor accuracy Poor precision More Examples!! Which number is more precise? (If you were buying a gold nugget, how many decimal places would you want to be sure of??) A) 3.00g B) 3.000g C) 3g ANSWER: B) 3.000g Which number is a more accurate measure of a 7.00kg block? (accuracy deals with closeness!!) A) 6.93kg B) 6.9kg C) 8 kg Off by: 0.07kg 0.1kg 1kg ANSWER: A) 6.93kg Remember to think about accuracy , precision and significant digits ALL THE TIME!! An answer of 9.067894038399L is not an acceptable answer in lab, test or any other piece of paper that bears the privilege of sporting your signature!! Percent Error A mathematical value representing the difference between the accepted value and the experimental value. (analyzes the accuracy of the data) Accepted value correct value (what the data should be – based on reliable research) (What you are told it should be!!) Experimental value value measured in lab (what you measure or calculate it to be!!) Formula: % Error = Experimental value – Accepted value x 100% Accepted value Negative % error Your answer is lower than it should be. Positive % error Your answer is higher than it should be. % Error Example 1 A block of wood has a length of 4.3cm. A student measures the length and finds it to be 4.4cm. What is this student’s % error? Accepted value – experimental value x 100% Accepted value Experimental value = 4.4 cm (student measured) Accepted value = 4.3cm (what you’re told it should be) 4.3cm – 4.4cm x 100% = -2.3 % error 4.3cm % Error Example 2 A block of wood has a length of 15.8cm. A student measures the length and finds it to be 15.7cm. What is this student’s % error? Accepted value – Experimental value Accepted value x 100% Experimental value= 15.7 cm (student measured) Accepted value= 15.8cm(what you’re told it should be) 15.8cm – 15.7cm x 100% = 0.63 % error 15.8cm Metric Measurements SI (International System of units) system is used worldwide based on units of ten. Base Unit Abbreviation Meter m Second s Kelvin K Gram g Ampere A Candela Cd Mole Mol Quantity Measured Length Time Temperature Mass Electric current Luminous intensity Amount of substance All other units are derived from the seven base units (thus known as derived units). Volume: The amount of space an object occupies. Can be determined by three methods: 1. Formula: volume=ℓ x w x h (units= cm3) 2. Measuring with a graduated cylinder (units = mL) 3. Determined by water displacement (units = mL = cm3) Remember that 1 mL = 1 cm3 If a metal block displaces 8.0mL of water, then the block’s volume in cm3 is 8.0cm3. * What is the volume in cm3 of a metal toy that displaces 7.5mL of water when dropped into a small container? 7.5cm3 Mass A measure of the amount of matter in an object. Measured on a balance. (ex. Triple beam balance, digital or electronic balance) Weight A measure of the force of gravity acting on an all objects with mass. The product of mass and gravitational force. Density The amount of mass per unit of volume. formula: density=mass/volume D = m/V Temperature A measure of the average kinetic energy of the particles in a sample of matter. The scales are used to measure temperature are Fahrenheit, Celsius and Kelvin. Kelvin is the SI unit for temperature. Using Prefixes The distance form Maryland to Utah would be best measured in km. The length of a pencil would be measured in cm. The height of the letter “h” would be measured in mm. ** Different prefixes are used for different amounts** Prefix Mega Kilo Hecta Deka Base unit Deci Centi Milli Micro Nano Pico Symbol M k h dk d c m μ n p # base units One million One thousand One hundred Ten One One tenth One hundredth One thousandth One millionth One billionth One trillionth Sci. Notation 1 x 106 1 x 103 1 x 102 1 x 101 1 x 100 1 x 10-1 1 x 10-2 1 x 10-3 1 x 10-6 1 x 10-9 1 x 10-12 Conversions To convert between units, you could move the decimal point. (if factors of ten!!) _____ ____ 0.0004 0.004 ____ 0.04 0.4 4 40 400 K h dk U d c m 5 50 500 5000 50000 _____ 500000 ______ 5000000 _____ Dimensional Analysis (factor-label method) used to convert from units 1) write down what is known (number & unit) 2) set up a conversion factor with the target end unit on top and one known unit on the bottom (if unit you are canceling is on top) 3) divide the product of the numbers in the numerator by the product of the numbers in the denominator 4) make sure that the final answer has the same number of significant figures as the number given. Examples #1. How many seconds are in 7 minutes? known = 7 min. conversion factor = 60 sec. / 1 min. 7 min x 60 sec = 420 sec = 420 sec 1 min 1 Examples #2. How many cm are there in 5.2 meters? known = 5.2 m conversion factor = 1 m / 100 cm 5.2 m x 100 cm = 520 cm = 520 cm 1m 1 Examples #3. How many hours are there in 3 weeks? known = 3 wk conversion factor = 1 wk / 7 days 1 day / 24 hr 3 wk x 7 days x 24 hr = 520 hr = 520 hr 1 wk 1 day 1