supporting information-passivation paper-2013-09
advertisement

The Effect of Passivation on Different GaAs Surfaces
Ted H. Yu1, Liang Yan2, Wei You2, Ramesh B. Laghumavarapu3, Diana Huffaker3, Christian Ratsch1
1
Department of Mathematics, University of California at Los Angeles and Institute of Pure and Applied
Mathematics, Los Angeles, CA 90095, USA
2
Department of Chemistry, University of North Carolina, Chapel Hill
3
Department of Electrical Engineering, University of California at Los Angeles, Los Angeles, CA 90095,
USA
Experimental Detail:
PEDOT:PSS (poly(3,4-ethylenedioxythiophene) : poly(styrenesulfonate)) was deposited on GaAs wafers
of (111B, As terminated) and (100) orientations. Four different GaAs wafer samples were tested: (100)
and (111B) wafers both with and without sulfur passivation. Si-doped n-type GaAs wafer (carrier
concentration: 1×1018 cm-3 for (100), 1~5×1018 cm-3.for (111B)) with annealed AuGe/Ni/Au back ohmic
contact was cleaned by sequentially dipping in acetone and isopropanol for 5 minutes each. Then the
GaAs wafer was immersed in aqueous HCl solution (10% v/v) for 30 seconds to remove the native oxide.
After that, the passivated wafers were passivated by a two-stepS1 process with: 1) a 5 hour treatment of
30 mM octanethiol solution in ethanol followed by 2) a 15 minute treatment of 22wt% aqueous
ammonium sulfide solution. The prepared GaAs with or without passivation was spin coated with 5%
DMSO doped PEDOT:PSS (CleviosTM PH500) with Zonyl FSO-100 surfactant (Sigma-Aldrich, 5mg Zonyl
FSO-100 in 1 ml doped PEDOT:PSS) at 2500 RPM for 1 minute to get a thin PEDOT:PSS layer. A separate
ITO glass was cleaned by ultrasonication in deionized water, acetone and isopropanol sequentially for 15
minutes each and then dried under nitrogen flow and followed by the UV-Ozone treatment for 15 min.
The DMSO doped PEDOT:PSS was also spin coated on such cleaned ITO glass at 1200 RPM for 10
seconds. Then, the previously PEDOT:PSS/GaAs substrate was put facing down onto the ITO glass when
the PEDOT:PSS on ITO was still wet. The entire device (ITO/PEDOT:PSS/GaAs) was then dried in a
vacuum oven at room temperature for 3 hours. All processes were at room temperature without any
annealing process. The photovoltaic performance characterization of finished devices was conducted
with the solar simulator (Oriel 91160, 300 W) under one sun AM1.5 global condition (100 mW/cm2
calibrated by a NREL certified standard silicon cell) at room temperature in the glove box. Current
density vs. potential (J-V) curves under illumination and in dark were recorded with a Keithley 2400
digital source meter.
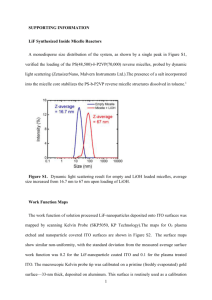
50
(111)B Nonpassivated
(111)B Passivated
(100) Nonpassivated
(100) Passivated
2
Current (mA/cm )
40
30
20
10
0
-10
-0.2 0.0
0.2 0.4 0.6
Voltage (V)
0.8
1.0
Surface
(100)
(100)
Passivation
No
Yes
Jsc(mA/cm2)
7.17
7.74
Voc (V)
0.33
0.51
FF
28%
48%
η
0.67%
1.91%
(111B)
(111B)
No
Yes
9.61
8.66
0.65
0.65
65%
68%
4.08%
3.84%
Figure S1. J-V curves under 1 sun AM 1.5 illumination
We fit the J-V curves in the dark of devices of GaAs/PEDOT:PSS with different orientation and
passivation condition to the modified Shockley equation with series resistance and parallel resistance
(Equation 1) to get the ideality factor n and saturated current Js.
𝐽=
𝑅𝑝
𝑞(𝑉 − 𝐽𝑅𝑠 )
𝑉
{𝐽𝑠 [exp (
) − 1] + }
𝑅𝑠 + 𝑅𝑝
𝑛𝑘𝑇
𝑅𝑝
(1)
The fitting result of dark J-V curves of devices of GaAs/PEDOT:PSS with different orientation and
passivation conditions.
Surface Passivation Js (nA/cm2)
n
(100)
No
2.54
1.70
(100)
Yes
0.93
1.44
(111B)
No
1.3e-3
1.15
(111B)
Yes
1.0e-3
1.12
Figure S2. Dark J-V curves of device of devices of GaAs/PEDOT:PSS with different orientation and
passivation condition.
Sulfur Binding
BC
CFH
DEG
EF
EG
FH
GI
HI
IJ
JK
Ebind,S
1.82 eV
0.34 eV
0.14 eV
0.33 eV
0.43 eV
0.50 eV
-0.29 eV
-0.14 eV
0.26 eV
0.71 eV
Sulfur Replacement
A
E
F
J
K
Ereplace,S
2.09 eV
0.56 eV
0.39 eV
0.68 eV
0.71 eV
CH₃CH₂S• Binding
AB
E
F
G
H
J
K
Ebind,CH₃CH₂SH
1.67 eV
0.12 eV
0.33 eV
0.54 eV
0.96 eV
1.13 eV
1.29 eV
Ebind,S = -EGaAs+S + ES(bulk) + EGaAs)
Ereplace,S = -EGaAs+S - EAs(bulk) + ES(bulk) + EGaAs)
Ebind,CH₃CH₂SH = -EGaAs+CH₃CH₂SH - ½EH₂ + ECH₃CH₂SH + EGaAs)
Figure S3. The binding and replacement energy of sulfur and CH₃CH₂S• as defined above for various
sites on the 2(2X4) - Defect D surface. For sulfur, replacement site A is most stable (2.09 eV). For
CH₃CH₂S•, binding site AB is most stable (1.67 eV).
(a)
(b)
Figure S4. Band structure and DOS after passivation by sulfur and thiol. Left panel represents the band
structure of (a) Sulfur Replacement Site A and (b) CH₃CH₂S• Binding Site AB with hybrid functional. The
right panel represents the corresponding density of states. C1 is the CBM state as visualized in Figure 4
with slight differences described in the text. For both cases, the V1 state disappears from the midgap.
References:
S1.
P. Arudra, G. M. Marshall, N. Liu and J. J. Dubowski, J Phys Chem C 116 (4), 2891-2895 (2012).