Diapositiva 1
advertisement
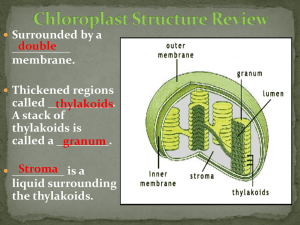
The principal function of the leaves is the production of nutritional substances through some reactions called photosynthesis. The photosynthesis lets produce glucose (C6H12O6) and oxigen (O2) starting from water (H2O) and carbon dioxide (CO2). The oxigen is released in the atmosphere. 6 CO2 + 6H2O ==> C6H12O6 + 6O2 The photosynthesis is carried out by the chlorophyll, thanks to sunlight. A second physiological activity that is done by leaves is represented by the transpiration (a loss of water in the form of steam). From the first years of the XX century the English botanist F.F.Blackman showed that there are two phases in the photosynthesic process. He noticed that the speed of photosynthesis increased with the light intensity, but this increase was influenced by the temperature. This observation was used by Blackman in order to conclude that, in the photosynthesic process, there are reactions which are determined by the presence of light and other reactions, which are determined by enzymes that lose their efficacy beyond a certain temperature. Nowadays we know that the photosynthesis reactions develop in two phases. In the first one, which is influenced by light, the daylight hits some molecules of chlorophylle A. The molecules of chlorophyll A lose one electron which is used to make energy. In this phase also the molecules of water become ion H+, gaseous oxigen and electrons which replace the chlorophyll’s ones. In the second phase of photosynthesis without light the energy that has been produced in the previous phase is used to produce sugar. Besides the carbon dioxide is used to create a structure of other organic molecules. This process is called “Cycle of Calvin”. A system is a restricted group of matter, distinguishable from the surrounding environment. In every system there is one or more phases, which are portions of matter with the same features delimited by sharp and definite surfaces. A system with one phase is called homogeneous. If there is only one substance this is called pure, but if there are two or more phases, which are not identified with the traditional methods of observation it is called solution. When the system is composed by two or more phases it is called heterogeneous. MATTER HOMOGENEOUS SYSTEMS SOLUTIONS HETEROGENEOUS SYSTEMS PURE SUBSTANCES The most common techniques of separation used to divide the different components of the systems are: Decantation; Filtration; Centrifugation; Distillation; Crystallization; Extraction with solvents; Chromatography. The extraction with solvent is usually used to divide the solutions. This technique is based on the affinity of a solute with another solvent different from the solvent in which it is dissolved. In order to extract the substance dissolved into the solution we can use a solvent which has a more affinity with this substance. The only aspect that we have to take into consideration is that the two solvents aren’t soluble among them because in this way the solution becomes more complex. The chromatography is the most versatile among the separation techniques. It was created in 1906 by the Russian botanist Michael Tswett, this technique doesn’t differ from the simple extraction, but multiplies its efficacy. The solvent, that in this case is called mobile phase conveys the components of the system through a fixed phase. In the chromatography on layer, the fixed phase, composed by a thin coat of inert matter such as silica gel or alumina, is fixed on a sheet of aluminium. A thin line is drawn and some drops of solution are put on it. This zone is called sowing zone. Then a small part of the sheet is immersed in a container in which there is another solvent (for example water, acetone, hexane…)so that the initial solution moves through the fixed phase due to the capillary action; as the various substances of the solution move at different speeds they separate. Take some leaves of spinach, break into bits and put them into the mortar with some sand to facilitate the crumbling. Crush and pour some alcohol. Add some calcium bicarbonate with the spatula Pick up a small quantity of solution with capillary. Take a sheet of aluminium with silica gel and draw a line with a pencil Sow some drops of the solution on the line. Place the sheet upright into the pot and close it immediately so that the solvent doesn’t evaporate. Wait until the solvent goes up the whole sheet carrying the pigments. When the solvent reaches the top of the sheet take it out and wait so that the solvent evaporates. YELLOW – ORANGE band: carotenoids GREEN- BLUE band: chlorophyll b GREEN- YELLOW band: chlorophyll a LEMON YELLOW band: xantophyll SOWING ZONE During the experiment we can note a solid and a liquid phase, in fact when we pour the alcohol in the mortar we obtain a solution of pigments and alcohol . After the evaporation of the solvent it is possible to see the separation of the pigments of spinach. The pigments are arranged in this way: PIGMENT DISTANCE FROM SOW LINE Xantophyll 1,8 cm chlorophyll a 2,2 cm Chlorophyll b 2,4 cm Β carotene 5,6 cm The leaves can catch the sunlight thanks to the pigments. The chlorophyll is the most important chloroplast and it is contained in the membrane of thylakoids. There are two kinds of chlorophyll: chlorophyll A which changes bright energy into chemical energy. A lot of cells also contain chlorophyll B that has a different colour. Usually there is a second group of pigments that are orange or red: the carotenoids. Normally they are masked by the green of chlorophyll because in the leaves there is more chlorophyll. In autumn the chlorophyll is the first pigment that disappears and the carotenoids become visible. The chlorophyll is fundamental for the photosynthesis. The chlorophyll molecule is composed of two parts. The first one, called “head”, intervenes in the light phase and takes part into the photochemical transformations; it is composed of a ring in which there is an atom of magnesium (Mg). The second part is an hydrocarbon tail linked to the first part and it has the function of introducing the chlorophyll into the thylakoids. This molecule is composed of a long hydrocarbon chain that ends with two rings of carbon atoms. The classical red of carotenoids is the direct consequence of the molecular structure of these mixtures. The elements composing the chain present some bonds among them which consent the structure to change its position and so let that the light spectrum changes and the colour of the pigments changes too. The bright energy can be used by the living beings only if it is first absorbed. The substances that can absorb the light are called pigments. Some pigments take up all the wavelengths of light therefore they appear black. Other pigments only absorb some wavelengths so they reflect those that they don’t absorb. Through this experience we have learnt that although the colour of leaves is determined by the union of several pigments, the chlorophyll prevails. It is for this reason that the only colour we can almost always see is green. . Through chromatography it is also possible to determine which pigments are contained into the leaves cells.