File - chohan`s chemistry
4.3 Measuring enthalpy changes
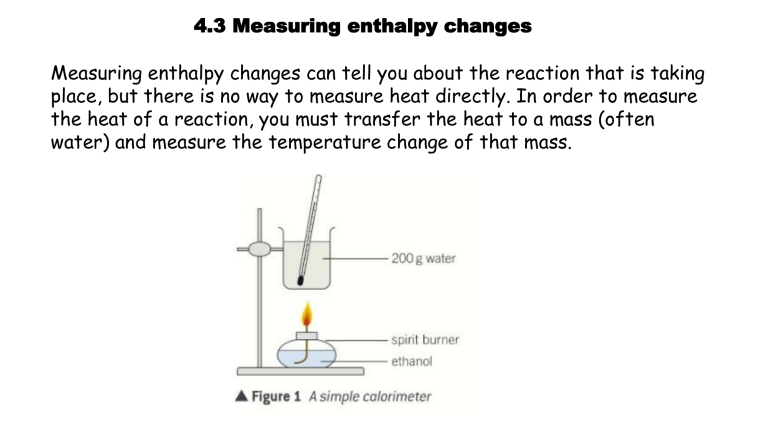
Measuring enthalpy changes can tell you about the reaction that is taking place, but there is no way to measure heat directly. In order to measure the heat of a reaction, you must transfer the heat to a mass (often water) and measure the temperature change of that mass.
DEFINITIONS IN ENTHALPY CHANGES
At A level it is now important that enthalpy changes are given names, other than just defining the reaction as being either exo or endothermic.
For example we can have:
1. The enthalpy change of formation ( D H O f
)
2. The enthalpy change of combustion ( D H O
C
)
The STANDARD MOLAR ENTHALPY OF FORMATION ( D H O f) = is the enthalpy change when 1 mole of a substance is formed from its constituent elements when all the reactants and products are in their standard states under standard conditions)
The STANDARD MOLAR ENTHALPY CHANGE OF COMBUSTION
( D H O c) = is the enthalpy change when 1 mole of a substance is burned completely in oxygen under standard conditions where all the reactants and products are in their standard states under standard conditions.
EXAMPLES:
1. Give an equation to show the D H O f
ANSWER:
C(s) + O
2
(g) CO of CO
2
(g)
2
2. Give an equation to show the D H O f
ANSWER:
2Al(s) + 1½O
2 of Al
(g) Al
2
O
2
3
O
3
(s)
3. Give an equation to show the D H O c of CH
3
ANSWER:
OH
CH
3
OH(l) + 1½O
2
(g) CO
2
(g) + 2H
2
O(l)
4. Give an equation to show the D H O c of potassium
ANSWER:
K(s) + ¼O
2
(g) ½K
2
O(s)
Do the worksheet on Definitions
HEAT AND TEMPERATURE
Temperature is related to the average kinetic energy of the particles in a system
As particles move faster the average KE increases and the temperature goes up.
It does not matter how many particles there are, temperature is independent of the number of particles
Temperature is measured with a thermometer
Heat is a measure of the total energy of all the particles present in a given amount of substance.
It does depend on how much substance is present
So a bath full of warm water will have more heat than a red hot nail because there are more particles in it
Heat always flows from high to low temperature
So heat will flow from the nail to the bath water, eventhough the water has more heat than the nail.
MEASURING THE ENTHALPY CHANGE OF A REACTION
There is no instrument which measures heat change directly
To measure heat change you must allow the heat to be transferred into a particular mass of substance, often water.
Then you need to know 3 things to be able to measure D H O :
1. Mass of substance that is being heated or cooled
2. Temperature change
3. Specific heat capacity of the substance
A SIMPLE CALORIMETER
A certain mass of fuel is burned to heat a known mass of water placed in a colorimeter (copper can).
The temperature rise of the water is measured using a thermometer.
The equation below is used to calculate the molar enthalpy change of combustion for the fuel in Joules
In joules In grams
In Jg -1 K -1
For water this value is 4.2Jg
-1 K -1
In Kelvin
The specific heat capacity is the amount of heat needed to raise the temperature of 1g of a substance by 1K.
REMEMBER – that D H O c is measured in kJmol -1 and NOT joules so once we have used the equation q = mc D T
The answer we have for q (in Joules) will have to be converted into kJmol -1 .
WORKED EXAMPLE
0.32g of CH3OH (methanol) burns and causes the temperature of
200cm3 of water by 4K. Calc. the D H O c.
ANSWER q = mc D T
200 x 4.2 x 4
= 3360J
= 3.360kJ
Moles = m/Mr
÷
1000
= 0.32/32 = 0.01 mol
D H O c
= 3.36/0.01
= -336kJmol -1
0.16 g of methane (CH
4
) was completely combusted, under standard conditions to cause a 21.2°C temperature rise in 100 g of water.
ANSWER q = mc D T
= 100 x 4.2 x 21.2
= 8904J
= 8.904kJ
Moles = m/mr = 0.16/16 = 0.01mol
∴ D H O c = 8.904/0.01 = -890kJmol -1
MORE QUESTIONS
1. 0.92 g of ethanol (C
2
H
5
OH) was completely combusted, under standard conditions to cause a 65.3
° C temperature rise in 100 g of water. What is the standard enthalpy of combustion for methane?
2. 0.108 g of pentane (C
5
H
12
) was completely combusted, under standard conditions to cause a
50.3
° C temperature rise in 25.0 g of water. What is the standard enthalpy of combustion for methane?
3. 0.32 g of methanol (CH
3
OH) was completely combusted, under standard conditions to cause a
16 ° C temperature rise in 50 g of water. What is the standard enthalpy of combustion for methane?
A simple colorimeter of this type can be used to compare the D Hc values of a series of similar compounds because the errors will be similar foe every experiment.
However you can improve the results by cutting down the heat loss as shown:
THE FLAME COLOURIMETER
This type of colorimeter is designed to reduce heat losses.
The spiral chimney is made of copper
The flame is enclosed
The fuel burns in pure oxygen rather than air
Calculating experimental errors
Measurement error is defined as the disagreement or difference between a measurement and the true or accepted value.
Percentage error is a more useful concept. It gives you an idea of how large an error is relative to the measurement being taken. For example, if the error of a thermometer is ±0.5 °C, then this is quite small if the temperature being measured is 78.0 °C.
The percentage error is 0.5/78 x 100 = 0.6%
But if the temperature being measured is 5.0OC then the relative error would be much bigger.
The percentage error is 0.5/5 x 100 = 10%
In order to determine the enthalpy change when 1 g of ethanol is burnt, a student carried out the following procedure.
1. 100.00 g of water was placed in a copper calorimeter. The starting temperature of the water was recorded.
2. A spirit burner containing ethanol was placed on a digital balance and zeroed.
3. The copper calorimeter was then clamped above the spirit burner.
4. The wick was ignited under the copper calorimeter. As the water was heated it was continuously stirred.
5. When the mass of ethanol had decreased by 1.00 g, the flame was extinguished.
6. When the temperature had stopped rising, the final temperature was recorded.
The student calculated the heat change, q using the following equation.
The specific heat capacity of water is 4.18 J g −1 K −1 .
q = mass of water specific heat capacity of water rise in temperature
= 100 4.18 22
= 9.2 kJ
Evaluation of errors
How certain can we be that 9.2 kJ g −1 is accurate? This value depends on several measured quantities: namely the temperature of the water, the mass of water being heated and the mass of fuel being burnt.
Each measured quantity is itself subject to error, but what will be their individual effects on the overall error?
One way of comparing the relative error of each measurement is to calculate the percentage error:
Error in a measurement is half the value of the smallest division.
Or, in the case of digital instruments, the error is ± 1 the last digit of a digital measurement.
The measuring apparatus used in the experiment were:
digital balance, error: ± 0.01 g
mercury thermometer, error: ± 0.5
° C
Questions
1. Calculate the percentage error when measuring the mass of water. (1
mark)
2. Calculate the percentage error when measuring the mass of fuel.
(1
mark)
3. Calculate the maximum percentage error when measuring the initial temperature.
(1 mark)
4. Which of these measurements has the biggest effect on the overall error?
(1 mark)
5. How could the percentage error in this measurement be reduced? (2 marks)
The overall apparatus error can be calculated by adding the percentage errors.
6. Calculate the overall apparatus error.(2 marks)
7. The aim of this procedure was to determine an enthalpy change for the combustion of 1g of ethanol. The accepted value is 29.7kJ g −1 .
Discuss whether the overall apparatus error accounts for the difference between the accepted value and the experimental value.
8. List some other possible reasons, other than measurement errors, for the difference between the experimental value and the accepted value.
(2 marks)
PRACTICAL ON DHO
MEASURING ENTHALPY CHANGES OF REACTIONS IN
A SOLUTION
When solution react heat is generated in the solutions themselves and only has to be kept in the colorimeter
Expanded polystyrene beakers are often used as colorimeters.
These are good insulators (this reduces heat loss through their sides) and have a low heat capacity so they absorb very little heat
The specific heat capacity of solutions is usually taken to be the same as water i.e. 4.2Jg
-1 K -1 .
NEUTRALISATION REACTIONS
ACID + ALKALIS SALT + WATER
Neutralisation reactions usually give out heat energy as they are exothermic reactions
WORKED EXAMPLE
ANSWER q = mc D T
= 100 x 4.2 x 6.6
= 2772J ≡ 2.772kJ
No. of moles of acid (same as moles of alkalis) = cV = 1 x 50/1000 = 0.05mol
D
H O neut = 2.772/0.05 = -55kJmol -1
DISPLACEMENT REACTIONS
EXAMPLE
Zn(s) + CuSO
4
(aq) ZnSO
4
(aq) + Cu(s)
Zinc displaces copper. This reaction is exothermic.
EXAMPLE - 0.5g of zinc was added to 25.0cm
3 of 0.20moldm
-3 copper sulphate solution. The temperature rose by 10K.
Calc. the enthalpy of reaction D H O r.
ANSWER q = mc
D
T
= 25 x 4.2 x 10
= 1050J ≡ 1.050kJ
Moles of Zn = 0.5/65.4 = 0.0076mols
Moles of copper sulphate = cV
= 0.20 x 25/1000
= 0.005mol
This means that Zn was in excess; 0.05mol of each reactant takes part in the reaction, leaving some unreacted zinc behind.
1 mole of Zn would produce = 1.050/0.005 = -210kJmol -1
The negative answer is because the reaction is exothermic.
ALLOWING FOR HEAT LOSSES
Eventhough polystyrene is a good insulator, some heat will be lost from the sides and top leading to low values for enthalpy changes measured by this method.
This can allowed for by plotting a cooling curve.
As an example lets looks at the heat of neutralisation of HCl with NaOH.
METHOD
QUESTION – Using this reactions data calc. the D H O neut.
ANSWER: -58kJmol -1