File
advertisement
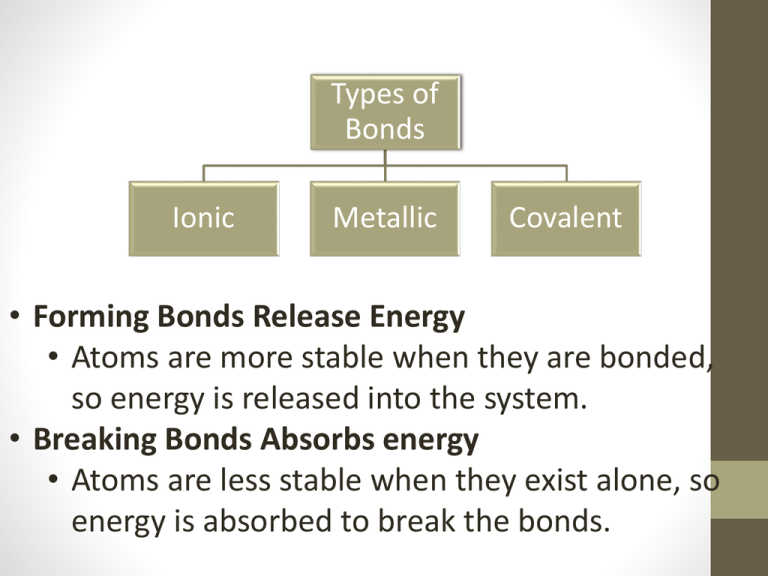
Types of Bonds Ionic Metallic Covalent • Forming Bonds Release Energy • Atoms are more stable when they are bonded, so energy is released into the system. • Breaking Bonds Absorbs energy • Atoms are less stable when they exist alone, so energy is absorbed to break the bonds. Ionic Compounds Types of bonding Ionic Metallic • Ionic Compounds: • Are usually composed of metal cations and non metal anions • Although they are composed of ions they are electrically neutral (no charge) Covalent Ionic Bonds Types of bonding Ionic Metallic • Opposite charges attract • Cations(+) and Anions (-) come together to form ionic bonds. • They are not actually bonded, the electrostatic forces (positive and negative charges) are drawn together. Covalent Table Salt… Really? • Watch this Na+[Cl-] in water • Now watch this Na Atom in water Types of bonding Ionic Properties of Ionic Compounds • Most Ionic compounds are crystalline solid at room temperature. • Exist as 3D-patterns called a lattice • Ionic Compounds are stable because of the balance between positive (+) charges and negative (-) charges. • Ionic compounds generally have high melting points. • When melted or dissolved in water ionic compounds conduct an electric current. Metallic Covalent Ionic Bonding: Thinking Deeper Types of bonding Ionic • Using valance electrons you can see how ionic bonds are created. Na Cl Metallic Covalent Puzzle Pieces of the World Types of bonding Ionic • What would you do if charges differed? K S Ca HCO Metallic Covalent How does differing charges work? S K K Types of bonding Ionic Metallic Covalent Try it! • Lithium Oxide • Barium Chloride Types of bonding Ionic Metallic Covalent Types of bonding Using Words to Create Letters • What if you are given names of ionic compounds? What would you do? • Would you call your mom? • Would you ask Siri? • Would you refer to the NYS Chemistry Reference Table (2002, 2006, 2011 edition)? • GO TO TABLE____ Ionic Metallic Covalent Types of Bonds Ionic Metallic Covalent Metallic Bonding • Metals are made up of closely packed cations rather than neutral atoms. • The valance electrons of metal atoms can be modeled as a sea of electrons • This means that valance electrons are mobile and are free to drift from one part of the metal to the next Types of Bonds Ionic Metallic Covalent Properties of Metallic Bonding Types of Bonds Ionic Understanding the “Sea of electrons” will explain the properties of this type of bond. • Metals are: • Good conductors of electricity • Because electrons (negative particles) are able to flow freely Metallic Cova Properties of Metallic Bonding (II) Types of Bonds Ionic Metallic • Metals are ductile (formed into wire) and malleable (hammered or bent without breaking) • Because the positive and negative charges flow freely they do not repel Covalent


