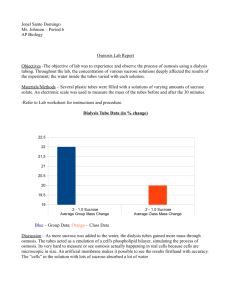
Biology 12*Osmosis Lab (Sucrose)
advertisement

Biology 12—Osmosis Lab (Sucrose) Purpose: In this experiment you will use dialysis tubing to investigate the relationship between solute concentration and the movement of water through a selectively permeable membrane by the process of osmosis. Materials (per group) 1) two 30 cm pieces dialysis tubing 2) 40 mL of assigned sucrose solution 3) 100 mL of unknown concentration sucrose solution 4) 25 mL graduated cylinder 5) funnel 6) thread 7) two 250 mL beakers 8) paper toweling 9) electronic balance Procedure: 1) Obtain two 30 cm long pieces of pre-soaked dialysis tubing. 2) Tie off one end of each piece with thread to form a bag. Tie tightly. Use a funnel to pour 20 mL of ONE of the following solutions into each bag. (a solution will be assigned to you) You will have two identical dialysis bags. a. 0 M b. 0.5 M sucrose c. 1.0 M sucrose d. 1.5 M sucrose e. 2.0 M sucrose 3) Remove most of the air from the bags by drawing the dialysis bags between two fingers. Tie off the other end of the bag leaving sufficient space for the expansion of the contents. 4) Use a graduated cylinder to measure out 100 mL of water and add to a 250 mL beaker. DO NOT JUST USE THE MEASUREMENT ON THE SIDE OF THE BEAKER. Repeat with the unknown sucrose solution and your other 250 mL beaker. 5) Thoroughly blot the outside of the bags with a paper towel and find the initial masses. RECORD. 6) Immerse one bag in each beaker and allow to stand for 20 minutes. Swirl every 5 minutes. 7) At the end of 20 minutes, remove the bags from the beakers. Thoroughly blot the outsides to remove excess water and determine the final masses. RECORD. 8) Record your results on the board. You will obtain the results for the other four solutions from your classmates. 9) Use the following formula to calculate percent change in mass for your data table: % change final mass initial mass 100 initial mass Data & Observations -include qualitative (descriptive) observations -ex: the bag increased in mass in the known solution and decreased in mass in the unknown -include quantitative data in your recopied version of the following table: Contents of dialysis tube Initial mass (g) Water ?M Final mass (g) Water ?M Percent change in mass (%) Water ?M 0M 0.5 M 1.0 M 1.5 M 2.0 M Analysis: Answer the following in detail, in complete sentences in your formal report. 1) Plot the relationship between sucrose molarity (Y-axis) and % change in mass (X-axis). Do this on an entire sheet of graph paper. Note: Plot both lines on the same graph! Ie 2) Why do some dialysis bags lose mass? Use appropriate vocabulary terms in your answer. 3) Why do some dialysis bags gain more mass than others? 4) Use your graph to find the molarity of the unknown sucrose solution. Hint: what should the change in mass be if the two solutions are the same strength? Conclusion: -summarize the main points of theory that his lab demonstrates -explain how you were able to find the molarity of the unknown solution. -describe any sources of error you may have encountered in this lab. -explain any unusual results Name Block Science Lab Checklist Use and Attach this to the front of each lab report Organization 1. Name, Block, Date, Lab Partner in top right hand corner of page 2. Lab is typed or written neatly in blue or black pen 3. Lab report is sequenced in same order as this checklist, each section is followed by an empty line, section headings are underlined. 4. Title describes what lab is about and is underlined. Lab Question 5. Clearly states what we are trying to find out in the lab. Hypothesis 6. Directly answers lab question based on what you believe. 7. Well written in a complete sentence. Use third person. Materials 8. All materials are included (must be written out) 9. Organized in a bulleted or numbered vertical list format Procedure 10. Written in the following format: “As listed on page _____ in (book name or name of lab handout), steps ______ to _______” 11. Describes any changes made to given procedure Data & Observations 12. Data table has appropriate title and each column heading has a title. 13. Units are included (at top of column heading if appropriate) 14. All observations are recorded as requested 15. Each observation refers to a procedure step number 16. All work is shown for calculations, numbers are formatted correctly (usually rounded to 1 decimal place). 17. Diagrams are formatted correctly (refer to diagram format handout) Graph (If included) 18. Title includes plotted variables (dependent, y, versus independent, x). 19. Each axis has its own separate title, numbers clearly stated, & units clearly stated. 20. Legend is present (if more than one item shown) 21. Appropriate scale is used (graph uses MOST of GRAPH PAPER) 22. Data is plotted correctly. Correct variables are on correct axis 23. Best fit line is used. Analysis 24. All answers are in complete sentences 25. All work is shown with formulae and units for any necessary calculations Conclusion & Sources of Error 26. Hypothesis is clearly repeated; for example “The hypothesis that...” 27. Reasons to support of reject the hypothesis are given (should be from data) 28. Sources of error are included. 29. Sources of error explain HOW they may have affected the observations. Check if done Teacher feedback