The UWCCC Molecular Tumor Board (MTB)
advertisement

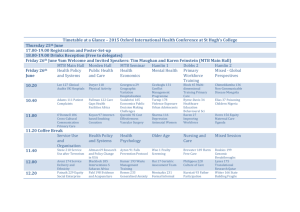
The UWCCC/WON Molecular Tumor Board (MTB) & Registration Protocol Mark E. Burkard MD PhD WON fall meeting October 24, 2015 “I shall never be content until the beneficent influence of the University reaches every home of the state.” -Charles Van Hise 1904 Launched September 2015 UWCCC/WON Molecular Tumor Board Genetics experts oncologists, pathologists, pharmacologists, pharmacists, cancer researchers Recommendations • Clinical trial • Off-label treatment • Standard treatment (not targeted) Submit pathology or genomics analysis MTB Chairs & Collaborators UWCCC WON Mark Burkard, Dusty Deming & Josh Lang Gundersen Lutheran/Lacrosse Dr. Ben M. Parsons Research Jill Kolesar & Anne Traynor Laboratory Science Bill Rehrauer & Jennifer Laffin Pathology Kristina Matkowskyj & Darya Buehler St. Vincent/GB Oncology Dr. Ruth Warren What to submit Clinical case information: • • • • • • Summary (age, diagnosis, stage, clinical status) Prior treatments for metastatic cancer Measurable disease (Y/N) Sample being tested Other clinically relevant information (clinical trial eligibility) Specific question Specimen and/or molecular data: • Specimen for genomic testing • Genomic test report What you get Recommendation: – Clinical trials – Off label use of targeted drugs* – No recommendation *Recommendations will adhere to an dynamic guidance document that specifies when off-label therapies can be recommended. MTB vs. Registration Protocol • MTB: – Expert advice on precision medicine – Commitment to research and using the best clinical evidence • Registration Protocol: – Research resource with genomic, clinical and outcome data – Available to WON members The WON MTB Workflow UW Collaborative Genomics Core UWHC clinical labs William Rehrauer Deaprtment of Pathology Request UW Pathology review/genomic sequencing The WON MTB Workflow The UWCCC Molecular Tumor Board MTB Meetings When: Where: 1st and 3rd Thursday of each month 4:30pm Who: Molecular tumor board chairs, trainees, WON collaborators, presenting physicians (optional) Present up to 4 cases, submitted 10 d prior Recommendation returned to treating physician What: Cost: UWCCC or Web-based Conference $0 … but enroll patient in registry Cases are de-identified for presentation The UWCCC MTB Registration Protocol The MTB Registration Protocol • To determine the frequency of acceptance of MTB clinical recommendations • To evaluate effectiveness of precision targeted therapies The MTB Registration Protocol • Open to all pts > 18 yo with histologically confirmed solid or hematological malignancy who will have genetic testing • Signed consent form • All molecular tumor profiling laboratory reports allowed Recruitment to the MTB Registration Protocol • Presented and consented to subjects during regularly scheduled clinic appointments • Subjects are registered into the UWCCC OnCore Database • Baseline and follow up clinical, genetic and demographic information collected and stored in OnCore Recruitment to the MTB & Registration Protocol Baseline Visit Follow-up Visits while on MTB molecularly-targeted Meeting therapy (Days 14-28) (if any)b Informed Consent X Collect required data elements X X Obtain tumor tissue for genomic testing X Xc Order genomic testa X Xc Order MTB X Obtain DNA blood sample for Lang Lab, path slides for Kolesar Lab, and tissue ford Deming Lab (if applicable) X Query Wisconsin Cancer Registry Annually X Archived Tumor for the MTB Registration Protocol • • Formalin-fixed paraffin block (preferred) OR Five- 5 micron sections placed on positively charged slides Slides should be labeled in a solvent-resistant manner with subject ID and slide numbers Allow slides 1 and 3 to air dry for ~24 hrs prior to shipping Slide 2 should be H&E stained and cover slipped prior to shipping Slides 4 and 5 should be unstained Blood Samples for the MTB Registration Protocol Prior to beginning targeted therapy: • • 2-10 mL Cellsave tubes 2-10mL EDTA tubes Formalin-fixed paraffin block (preferred) Samples will be processed for plasma and nucleated cells for analysis of cancer relevant targets and may be used to correlate with mutations observed in tumor tissue Timelines • MTB services are now available • MTB Registration Protocol available early 2016 How to access MTB and testing UW Collaborative Genomics Core • • • • • • www.med.wisc.edu/uwcgc Online Portal Access Portal Authorization Form Tests by Method Tests by Type Clinical Resources Genomic Research Resources The OutReach Online Portal • Hosted by the Wisconsin State Lab of Hygiene • Used to: Submit Requisition for Genomic Testing Request MTB Consultation View and print UWCGC Test Reports MTB Recommendations The OutReach Online Portal • The OutReach website address is https://www.slh.wisc.edu/elabs/Cytogenetics/ • OutReach can only be accessed by entering a valid user ID and password The OutReach Online Portal Click on “Orders” (bottom right) The OutReach Online Portal Click on “New Order” (lower left) The OutReach Online Portal Submitting MD is selected by clicking “Set Submitter” (alphabetical list of MDs is displayed) Prior pts and new pts can be accessed here The OutReach Online Portal Select “Order Type” (MTB) and select test to be ordered from the pull down MTB Prior pts and new pts can be accessed here Click “Place Order” MTB Contact Information Marissa Schuh, Coordinator mrschuh@uwcarbone.wisc.edu Dona Alberti, Administrator dba@uwcarbone.wisc.edu