Geometric Isomerism
advertisement

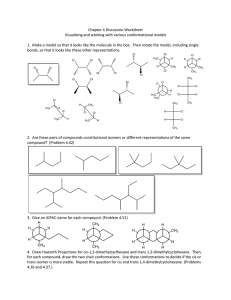
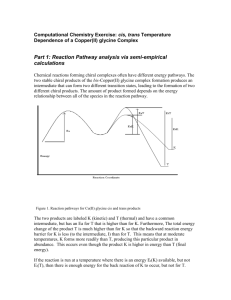
Title: Lesson 9 Geometrical Isomerism Learning Objectives: – Understand the term stereoisomerism – Understand and identify geometrical isomerism – Understand the chemical significance of cis-trans isomerism Molecular Modelling Make a molecule of but-2-ene Compare your model with your neighbours Main Menu Stereoisomerism Stereoisomers are compounds with the same structural formula but different 3D arrangement of atoms There are two types of stereoisomerism: Geometrical isomerism Optical isomerism Main Menu Geometrical (cis-trans isomerism) Geometric isomerism involves the arrangement of groups around a double bond Or a single bond that can’t rotate freely such as in a cyclic compound The substituted groups are fixed in space relative to each other. The position of the groups must be described with respect to a reference plane. The blue lines show the plane of reference for aliphatic and cyclic molecules… It happens because double bonds are not free to rotate. (Free rotation would allow the sideways overlapping p orbitals to move out of position, breaking the pi bond) Each C=C carbon must have two different groups attached to it. Main Menu cis-trans isomers When a molecule contains two or more different groups attached to the double bond or ring, they can be arranged to give two different isomers. • In the trans isomer, the substituents (the –CH3 groups) are on opposite sides of the double bond • In the cis isomer, the substituents are on the same side of the double bond Main Menu Cis–trans isomerism If an alkyl group or atom other than hydrogen is attached to each carbon then the isomers can be named either cis (‘on the same side’) or trans (‘on the opposite side’). cis-but-2-ene cis-1,2-dichloroethene 6 of 30 trans-but-2-ene trans-1,2-dichloroethene © Boardworks Ltd 2009 cis or trans? Note:You write the cis or trans as a prefix in italics… Main Menu Main Menu Limitations of cis–trans isomerism In more complex organic compounds, in which multiple hydrogens have been substituted by different groups, isomers cannot be defined using the cis–trans notation. For example, is it possible to identify which of these halogenoalkanes is the cis isomer and which is the trans isomer? Instead, a different system is used for these type of molecules: E–Z notation. 9 of 30 © Boardworks Ltd 2009 E–Z isomerism The E–Z notation is used to identify stereoisomers that cannot be called cis or trans. Isomers are identified as either E or Z depending on what ‘priority’ is given to the groups attached to the carbon atoms in the double bond. The priority of these groups is determined by a complex series of rules. 10 of 30 E represents the German word ‘entgegen’, and corresponds to trans isomers. The highest priority groups are on the opposite side of the double bond. Z represents the German word ‘zusammen’, and corresponds to cis isomers. The highest priority groups are on the same side of the double bond. © Boardworks Ltd 2009 E/Z isomerism So, in this example: Left side = Br (highest priority) Right side = C2H5 (highest priority) This makes a Z isomer… Rules of priority Rule 1: Look at the atom bonded to carbon of the double bond. The atom with the higher atomic number gets priority. Rule 2: If the atoms are the same, apply the rule to the next bonded atom. This means longer hydrocarbon chains have higher priority… Next, compare the positions of the highest priority groups on the two carbons of the double bond… If the two highest priority groups are on the same side = ‘Z’ isomer If the two highest priority groups are on the opposite side = ‘E’ isomer Main Menu Main Menu Physical Properties Cis and trans and E/Z isomers may have different physical properties depending on the influence of the substituent groups on polarity and shape and symmetry of the molecules. BP, MP and solubility data are reported separately for each isomer… Main Menu To do (individually, not pairs): Build, draw and label the cis and trans isomers of: Pent-2-ene 2,3-dichloro-but-2-ene 1,2-dichlorocyclopropane 1,2-dichlorocyclobutane Research the melting/boiling points of cis/trans but-2-ene, hex-3-ene and oct-4-ene. What pattern do you notice between the cis and trans isomers? Why do you think this happens? Draw the cis and trans isomers of 1,2-dichloroethene. Predict, with a reason, which you think will have the higher boiling points. Research online to determine whether you were right and think of a reason if you were wrong. Main Menu Chemical properties of geometric isomers Can be different For example: Why do you think this happens? Hint: think about the previous couple of lessons Main Menu Research Research online the importance of geometrical isomerism Find three examples of commercial compounds (medicines, plastics etc) where geometrical isomerism plays a key role in their properties Main Menu Key Points Cis isomers: the substituents are on the same side Trans isomers: the substituents are on opposite sides Cis alkenes have lower mp/bp than trans due to ‘rounder’ shape Cis halogenoalkenes have higher mp/bp due to polarity Chemical properties can be different where cis brings groups close enough to react Main Menu