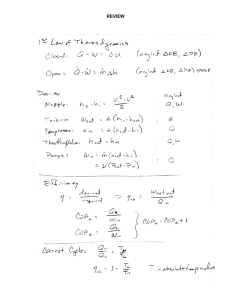
V2 T2 - Cloudfront.net
advertisement
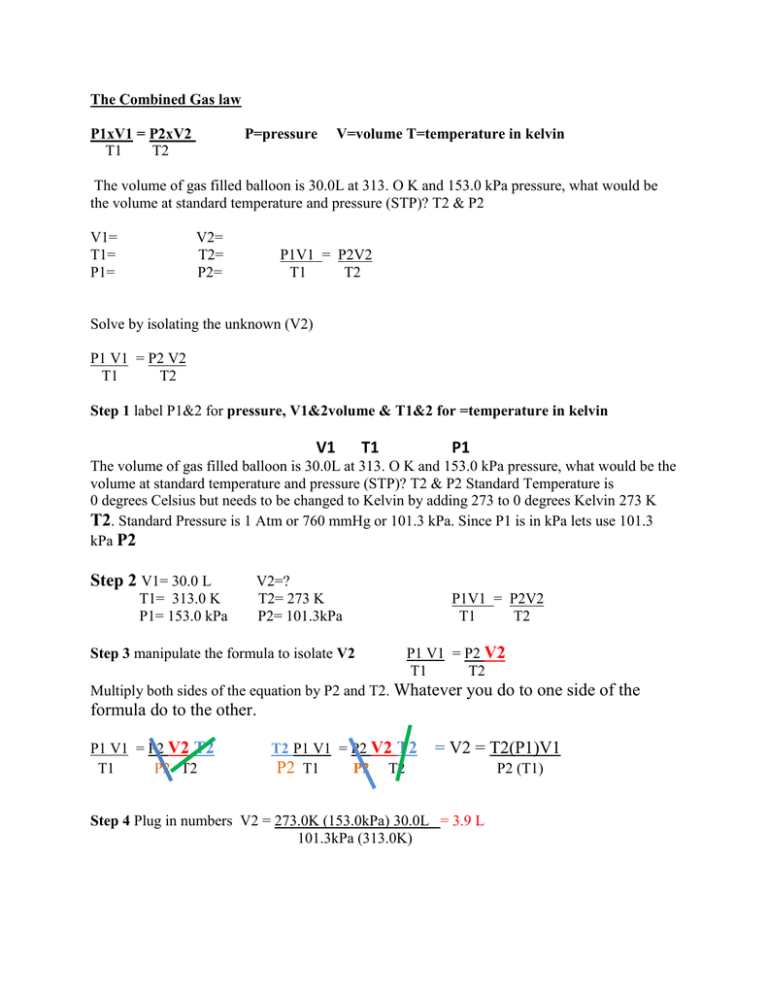
The Combined Gas law P1xV1 = P2xV2 T1 T2 P=pressure V=volume T=temperature in kelvin The volume of gas filled balloon is 30.0L at 313. O K and 153.0 kPa pressure, what would be the volume at standard temperature and pressure (STP)? T2 & P2 V1= T1= P1= V2= T2= P2= P1V1 = P2V2 T1 T2 Solve by isolating the unknown (V2) P1 V1 = P2 V2 T1 T2 Step 1 label P1&2 for pressure, V1&2volume & T1&2 for =temperature in kelvin V1 T1 P1 The volume of gas filled balloon is 30.0L at 313. O K and 153.0 kPa pressure, what would be the volume at standard temperature and pressure (STP)? T2 & P2 Standard Temperature is 0 degrees Celsius but needs to be changed to Kelvin by adding 273 to 0 degrees Kelvin 273 K T2. Standard Pressure is 1 Atm or 760 mmHg or 101.3 kPa. Since P1 is in kPa lets use 101.3 kPa P2 Step 2 V1= 30.0 L T1= 313.0 K P1= 153.0 kPa V2=? T2= 273 K P2= 101.3kPa Step 3 manipulate the formula to isolate V2 P1V1 = P2V2 T1 T2 P1 V1 = P2 V2 T1 T2 Multiply both sides of the equation by P2 and T2. Whatever you do to one side of the formula do to the other. P1 V1 = P2 V2 T2 T1 P2 T2 T2 P1 V1 = P2 V2 T2 P2 T1 P2 T2 = V2 = T2(P1)V1 Step 4 Plug in numbers V2 = 273.0K (153.0kPa) 30.0L = 3.9 L 101.3kPa (313.0K) P2 (T1)