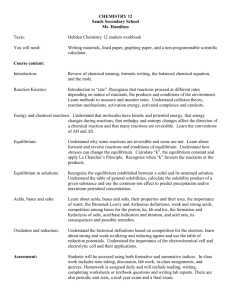
Organic Jeopardy
advertisement
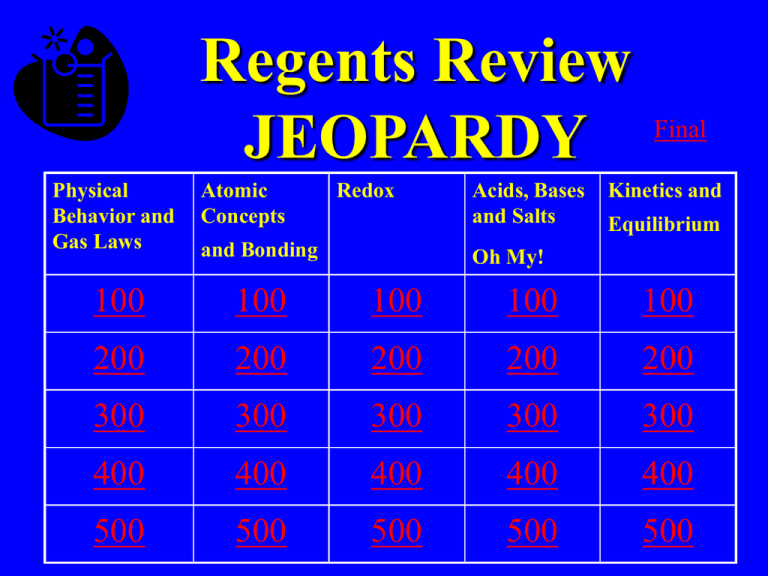
Regents Review JEOPARDY Physical Behavior and Gas Laws Atomic Concepts Redox and Bonding Acids, Bases and Salts Final Kinetics and Equilibrium Oh My! 100 100 100 100 100 200 200 200 200 200 300 300 300 300 300 400 400 400 400 400 500 500 500 500 500 Answer • Temperature measures this type of energy Question • What is kinetic energy? Answer • Substance that contains 2 or more different elements chemically combined Question • What is a compound? Answer • As pressure increases on a gas, volume does this Question • What is decrease? Answer • During a phase change this type of energy changes. Question • What is potential energy? Answer • These are 2 examples of substances that sublime Question • What are CO2 and I2? Answer • Atomic # always equals this subatomic particle Question • What is proton? Daily Double • How much do you wish to wager? Daily Double Answer • Region where electrons are found Double JEAPORDY Question • What is an orbital? Answer Answer • This type of bond is between 2 different nonmetals Question • What is covalent? Answer • This type of bonding affects phases/phase change Question • What are intermolecular? Answer • The type of bonding found in MgSO4 Question • What are covalent and ionic? Answer • These subatomic particles are gained and lost during redox reactions Question • What are electrons? Answer • Reaction where elements gain electrons Question • What is reduction? Answer • Ions flow through this in a voltaic cell Question • What is the salt bridge ? Answer • The element in K2Cr2O7 that has an oxidation # of +6 Question • What is chromium? Answer • In the reaction Zn +CuSO4 →ZnSO4 +Cu This element is undergoing oxidation Question • What is zinc? Answer • Acids, Bases and salts are considered this because they conduct in solution Question • What is an electrolyte? Answer • At this is the most soluble salt o 50 C Question • What is NaNO3? Answer • Increasing temperature has this affect on the solubility of gas Question • What is decrease? Answer • Salts are this type of compound Question • What is ionic? Answer • Arrhenius bases follow this rule Question • What is having solution? OH in Answer • Adding something causes a reaction to shift in this direction (in general) Question • What is away? Answer • When a reaction is at equilibrium these are equal Question • What is rate of forward and reverse reaction? Answer • This type of solution is at equilibrium Question • What is saturated? Answer • This value is the difference between the energy of the products and the energy of the reactants Question • What is heat of reaction? Answer • Increasing pressure shifts a reaction towards the direction of this Question • What is fewer gas molecules? Final Jeopardy • Organic Chemistry • This compound would be named ethyl pentanoate (DRAW!)