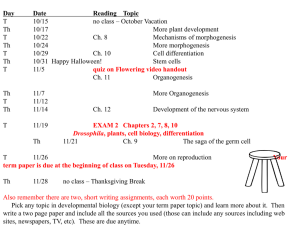
PPT - Arne Christensen | Anna Maria College
advertisement
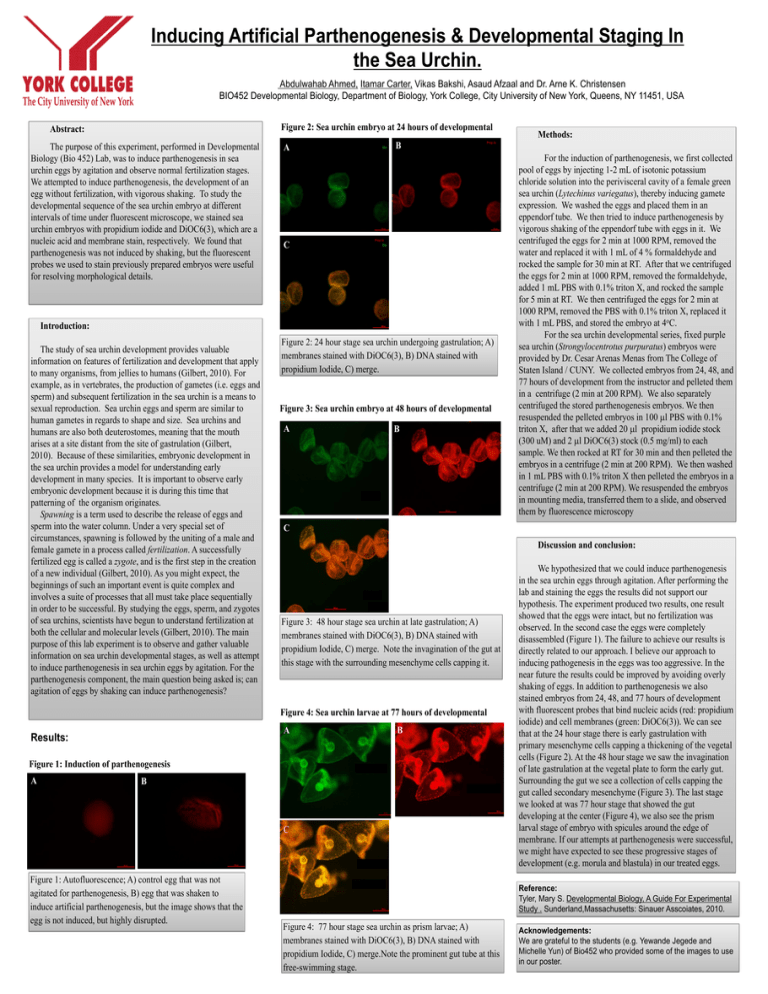
Inducing Artificial Parthenogenesis & Developmental Staging In the Sea Urchin. Abdulwahab Ahmed, Itamar Carter, Vikas Bakshi, Asaud Afzaal and Dr. Arne K. Christensen BIO452 Developmental Biology, Department of Biology, York College, City University of New York, Queens, NY 11451, USA Figure 2: Sea urchin embryo at 24 hours of developmental Abstract: The purpose of this experiment, performed in Developmental Biology (Bio 452) Lab, was to induce parthenogenesis in sea urchin eggs by agitation and observe normal fertilization stages. We attempted to induce parthenogenesis, the development of an egg without fertilization, with vigorous shaking. To study the developmental sequence of the sea urchin embryo at different intervals of time under fluorescent microscope, we stained sea urchin embryos with propidium iodide and DiOC6(3), which are a nucleic acid and membrane stain, respectively. We found that parthenogenesis was not induced by shaking, but the fluorescent probes we used to stain previously prepared embryos were useful for resolving morphological details. A B C Introduction: The study of sea urchin development provides valuable information on features of fertilization and development that apply to many organisms, from jellies to humans (Gilbert, 2010). For example, as in vertebrates, the production of gametes (i.e. eggs and sperm) and subsequent fertilization in the sea urchin is a means to sexual reproduction. Sea urchin eggs and sperm are similar to human gametes in regards to shape and size. Sea urchins and humans are also both deuterostomes, meaning that the mouth arises at a site distant from the site of gastrulation (Gilbert, 2010). Because of these similarities, embryonic development in the sea urchin provides a model for understanding early development in many species. It is important to observe early embryonic development because it is during this time that patterning of the organism originates. Spawning is a term used to describe the release of eggs and sperm into the water column. Under a very special set of circumstances, spawning is followed by the uniting of a male and female gamete in a process called fertilization. A successfully fertilized egg is called a zygote, and is the first step in the creation of a new individual (Gilbert, 2010). As you might expect, the beginnings of such an important event is quite complex and involves a suite of processes that all must take place sequentially in order to be successful. By studying the eggs, sperm, and zygotes of sea urchins, scientists have begun to understand fertilization at both the cellular and molecular levels (Gilbert, 2010). The main purpose of this lab experiment is to observe and gather valuable information on sea urchin developmental stages, as well as attempt to induce parthenogenesis in sea urchin eggs by agitation. For the parthenogenesis component, the main question being asked is; can agitation of eggs by shaking can induce parthenogenesis? Figure 2: 24 hour stage sea urchin undergoing gastrulation; A) membranes stained with DiOC6(3), B) DNA stained with propidium Iodide, C) merge. Figure 3: Sea urchin embryo at 48 hours of developmental A B Discussion and conclusion: Figure 3: 48 hour stage sea urchin at late gastrulation; A) membranes stained with DiOC6(3), B) DNA stained with propidium Iodide, C) merge. Note the invagination of the gut at this stage with the surrounding mesenchyme cells capping it. A B Figure 1: Induction of parthenogenesis A B C Figure 1: Autofluorescence; A) control egg that was not agitated for parthenogenesis, B) egg that was shaken to induce artificial parthenogenesis, but the image shows that the egg is not induced, but highly disrupted. For the induction of parthenogenesis, we first collected pool of eggs by injecting 1-2 mL of isotonic potassium chloride solution into the perivisceral cavity of a female green sea urchin (Lytechinus variegatus), thereby inducing gamete expression. We washed the eggs and placed them in an eppendorf tube. We then tried to induce parthenogenesis by vigorous shaking of the eppendorf tube with eggs in it. We centrifuged the eggs for 2 min at 1000 RPM, removed the water and replaced it with 1 mL of 4 % formaldehyde and rocked the sample for 30 min at RT. After that we centrifuged the eggs for 2 min at 1000 RPM, removed the formaldehyde, added 1 mL PBS with 0.1% triton X, and rocked the sample for 5 min at RT. We then centrifuged the eggs for 2 min at 1000 RPM, removed the PBS with 0.1% triton X, replaced it with 1 mL PBS, and stored the embryo at 4oC. For the sea urchin developmental series, fixed purple sea urchin (Strongylocentrotus purpuratus) embryos were provided by Dr. Cesar Arenas Menas from The College of Staten Island / CUNY. We collected embryos from 24, 48, and 77 hours of development from the instructor and pelleted them in a centrifuge (2 min at 200 RPM). We also separately centrifuged the stored parthenogenesis embryos. We then resuspended the pelleted embryos in 100 l PBS with 0.1% triton X, after that we added 20 l propidium iodide stock (300 uM) and 2 l DiOC6(3) stock (0.5 mg/ml) to each sample. We then rocked at RT for 30 min and then pelleted the embryos in a centrifuge (2 min at 200 RPM). We then washed in 1 mL PBS with 0.1% triton X then pelleted the embryos in a centrifuge (2 min at 200 RPM). We resuspended the embryos in mounting media, transferred them to a slide, and observed them by fluorescence microscopy C Figure 4: Sea urchin larvae at 77 hours of developmental Results: Methods: We hypothesized that we could induce parthenogenesis in the sea urchin eggs through agitation. After performing the lab and staining the eggs the results did not support our hypothesis. The experiment produced two results, one result showed that the eggs were intact, but no fertilization was observed. In the second case the eggs were completely disassembled (Figure 1). The failure to achieve our results is directly related to our approach. I believe our approach to inducing pathogenesis in the eggs was too aggressive. In the near future the results could be improved by avoiding overly shaking of eggs. In addition to parthenogenesis we also stained embryos from 24, 48, and 77 hours of development with fluorescent probes that bind nucleic acids (red: propidium iodide) and cell membranes (green: DiOC6(3)). We can see that at the 24 hour stage there is early gastrulation with primary mesenchyme cells capping a thickening of the vegetal cells (Figure 2). At the 48 hour stage we saw the invagination of late gastrulation at the vegetal plate to form the early gut. Surrounding the gut we see a collection of cells capping the gut called secondary mesenchyme (Figure 3). The last stage we looked at was 77 hour stage that showed the gut developing at the center (Figure 4), we also see the prism larval stage of embryo with spicules around the edge of membrane. If our attempts at parthenogenesis were successful, we might have expected to see these progressive stages of development (e.g. morula and blastula) in our treated eggs. Reference: Tyler, Mary S. Developmental Biology, A Guide For Experimental Study . Sunderland,Massachusetts: Sinauer Asscoiates, 2010. Figure 4: 77 hour stage sea urchin as prism larvae; A) membranes stained with DiOC6(3), B) DNA stained with propidium Iodide, C) merge.Note the prominent gut tube at this free-swimming stage. Acknowledgements: We are grateful to the students (e.g. Yewande Jegede and Michelle Yun) of Bio452 who provided some of the images to use in our poster.