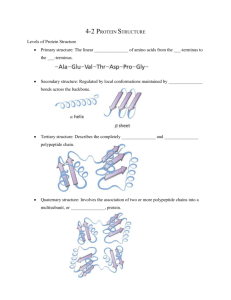
PowerPoint Presentation - Protein Structure Determination
advertisement
Protein Structure Determination Part 2 -X-ray Crystallography Para ver esta película, debe disponer de QuickTime™ y de un descompresor TIFF (LZW). Para ver esta película, debe disponer de QuickTime™ y de un descompresor TIFF (LZW). The method FT-1 FT Crystals X-rays Atoms EM versus x-ray • electron microcope • resolution ≈ 1nm • de Broglie wavelength of e- ≈ size of atom • transmitted light • lensing possible, 106x mag. • 2D image w/tilt • measures density. • sample is thin section • diffractometer • resolution up to 0.1nm = 1Å • wavelength ≈ size of atom • scattered light • no lens possible • 3D reconstruction • measures relative edensity • sample is single crystal X-ray diffractometer Experimental setup X-ray source X-ray detector beam stop Dimensions X-ray detector Beam width: ~0.20 mm Crystal thickness: 0.10-1.00 mm Unit cell: ~100Å = 0.00001mm Typical protein molecule: ~30Å = 0.000003 mm Dimensions C-C bond distance: 1.52Å N C CH3 O C Wavelength of Cu K X-rays: 1.5418Å Dimensions Angle of incidence= : 0-90° N C CH3 O C Bragg plane separation distance (resolution): 0.7-50Å N Dimensions Carbon atom C amount an electron moves in one xray cycle X-rays see e- as if they were standing still. Electromagnetic spectrum Wavelength of X-rays used in crystallography: 1Å - 3Å (Å = 10-10m) most commonly 1.54Å (Cu ) Frequency = c/ =(3x108m/s) /(1.54x10-10m) ≈ 2x1018 s-1 oscillating e- scatter X-rays …in all direction. oscillation e- emission Reflection planes •The “amplitude” of scattering is measured. •The amplitude is proportional to the differences of edensity in the direction of “reflection planes” •The orientation and separation of reflection planes is determined by the directions of the incoming and scattered rays. 10K+ reflections •Moving the X-rays and the detector gives a new set of planes. •Changing the angle of reflection changes the spacing (resolution). Reconstruction of e- density The density at every point in the crystal is calculated by summing over all of the density waves. Topics covering in this course • • • • • • Crystal growth Diffraction theory Symmetry Experimental methods Interpretation of data Software Equations you will need to know i e cos isin n 2dsin so s S x sym Mx v F hkl xyze 2 ihxky lz Euler's theorem Bragg's law Reciprocol space Symmetry Fourier transform xyz xyz F hkle2ihxky lz hkl Inverse Fourier transform How to know that you know • all terms defined • physical/geometric interpretation Supplementary reading Matrix algebra “An Introduction to Matrices, Sets and Groups for Science Students” by G. Stephenson ($7.95) Wave physics “Physics for Scientists and Engineers” by Paul A. Tipler Protein structure “Introduction to Protein Structure”-- by Carl-Ivar Branden and John Tooze “Introduction to Protein Architecture : The Structural Biology of Proteins” -- by Arthur M. Lesk Materials Gale Rhodes “Crystallography Made Crystal Clear” 3rd Ed. Academic Press graph paper straight edge protractor compass calculator w/trig functions http://www.bioinfo.rpi.edu/bystrc/courses/bcbp4870/bcbp4870.html