Chap. 7 - Quantum Chemistry
advertisement
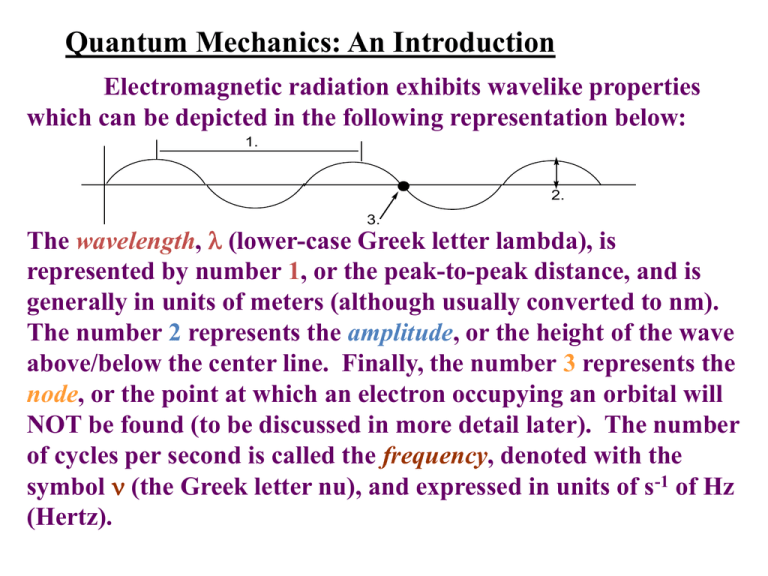
Quantum Mechanics: An Introduction
Electromagnetic radiation exhibits wavelike properties
which can be depicted in the following representation below:
1.
2.
3.
The wavelength, (lower-case Greek letter lambda), is
represented by number 1, or the peak-to-peak distance, and is
generally in units of meters (although usually converted to nm).
The number 2 represents the amplitude, or the height of the wave
above/below the center line. Finally, the number 3 represents the
node, or the point at which an electron occupying an orbital will
NOT be found (to be discussed in more detail later). The number
of cycles per second is called the frequency, denoted with the
symbol (the Greek letter nu), and expressed in units of s-1 of Hz
(Hertz).
Interference
• the interaction between waves is called
interference
• when waves interact so that they add to make a
larger wave it is called constructive interference
– waves are in-phase
• when waves interact so they cancel each other it is
called destructive interference
– waves are out-of-phase
2
Diffraction
• when traveling waves encounter an obstacle or opening
in a barrier that is about the same size as the
wavelength, they bend around it – this is called
diffraction
– traveling particles do not diffract
• the diffraction of light through two slits separated by a
distance comparable to the wavelength results in an
interference pattern of the diffracted waves
• an interference pattern is a characteristic of all light
waves
4
2-Slit Interference
6
Both light and waves can be
characterized by wavelength and frequency,
and all electromagnetic radiation moves at
the speed of light (c). Max Planck first
analyzed data from the emission of light
from hot, glowing solids. He observed that
the color of the solids varied with
temperature. Furthermore, Planck
suggested that a relationship exists
between energy of atoms in the solid and
wavelength. This led to the following
equation:
= c
= c
This equation has multiple interpretations.
If the wavelength is long, there will be
fewer cycles of the wave passing a point
per second; thus, the frequency will be low.
Conversely, for a wave to have a high
frequency, the distance between the peaks
of the wave must be small (short
wavelength). In summary, an inverse
relationship exists between frequency and
wavelength of electromagnetic radiation as
the speed of light is always constant.
Energy can be released (or absorbed)
by atoms in discreet chunks (also known as
quantum or photons) of some minimum
size; that is, energy is quantized and
emitted/absorbed in whole number units of
h. Collectively, we say that:
E = nh = nhc/
where
n = the quantum number, h = Planck’s constant
(6.626 x 10-34 J s), = frequency, = wavelength,
and c = speed of light (3.0 x 108 m/s). Energy (E)
is generally expressed in units of Joules (J),
where this actually implies J/atom.
Electromagnetic Spectrum
Electromagnetic radiation is produced by the
combination of electrical and magnetic fields which
are at right angles to one another (i.e. perpendicular).
The various types of waves (spectrum) include:
radiowaves, microwaves, infrared, visible light,
ultraviolet, x-rays, cosmic rays, and gamma rays.
All electromagnetic waves travel at the speed of
light; they differ from one another by their
corresponding wavelengths and frequencies.
Increasing Frequency () and Energy (E)
Radio
Micro
IR Visible UV X-ray Cosmic
Increasing Wavelength ()
Gamma
LIGHT
ELECTROMAGNETIC RADIATION
VISIBLE LIGHT IS ORDERED AS:
R
red
O
orange
700nm
low energy
long wavelength
Y
yellow
G
green
B
blue
I
indigo
V
violet
400nm
high energy
short wavelength
Example: A radiation source has a frequency of 2.35 x 1014 hertz,
what is the wavelength & energy associated with this light then
identify the type of radiation. How much energy & what frequency is
associated with a wavelength of 488.6 nm? Identify this radiation.
Amplitude & Wavelength
15
RADIATION SOURCE
TYPE OF INTERACTION WITH MATTER
X - RAY
energy transfer results in an inner shell electron being
ejected causing other electrons to cascade (emit) down to
lower levels. High energy & short wavelength.
UV/Vis
energy is transferred to outershell electron resulting in
the excitation of that electron which eventually cascades
back down to lower(ground) energy state.
Ir
energy is transferred to molecule causing the molecule to
vibrate; sometimes even lower energy is emitted back out
or the energy is lost as heat.
Microwave
energy is transferred to molecule causing the molecule to
vibrate and rotate; the higher initial energy is lost as
vibrational and rotational energy and as heat. Low
energy and long wavelength.
Workshop on EM Radiation
1. Yellow light exhibits a wavelength of approximately 570 nm.
Determine the frequency of this light and the total energy of the
photon being emitted in units of J and kJ/mol.
2. When an electron beam strikes a block of copper, x-rays with a
frequency of 2.0 x 1018 Hz are emitted. How much energy is
emitted at this wavelength by (A) an excited copper atom when it
generates an x-ray photon; (B) 1.00 mol of excited copper atoms;
and (C) 1.00 g of copper atoms?
3. A minimum energy of 495 kJ/mol is required to break the
oxygen-oxygen bond in O2. What is the longest wavelength of
radiation that possesses the necessary energy to break the bond?
4. Arrange the following types of photons of radiation in order of
increasing speed: infrared radiation, visible light, x-rays, and
microwaves. Which form of radiation possesses the longest
wavelength? Which form of radiation possesses the largest
amount of energy per photon?
Photoelectric Effect
Consider incident radiation which consists of a
stream of photons of energy h. Once these particles strike
the surface of a metal, the energy is absorbed by an
electron. If the energy of the photon is less than the energy
required to remove an electron from the metal, then an
electron will NOT be ejected. Moreover, the energy
required to remove an electron from the surface of a metal
is called the work function () of the metal. However, if
the energy of the photon is greater than the work function,
then an electron is ejected with a kinetic energy equal to
the difference between the energy of the incoming photon
and the work function. That is,
KEelectron = ½ mv2 = h - ho
where
m = mass, v = velocity, h = Planck’s constant, and = frequency.
Photoelectric Effect
These results, known as the photoelectric effect, can be
summarized alongside with Einstein’s theory:
1. An electron can be driven out of the metal only if
it receives at least a certain minimum energy, ,
from the photon during the collision. Therefore, the
frequency of the radiation must have a certain
minimum value if electrons are to be ejected. This
minimum frequency depends on the work function
and hence on the identity of the metal.
2. Provided a photon has enough energy, a collision
results in the immediate ejection of an electron.
3. The kinetic energy of the ejected electron from
the metal increases linearly with the frequency of the
incident radiation according to the equation ½ mv2 =
h - ho.
Practice Problems on EM Radiation & the Photoelectric Effect.
1. Yellow light is given off by a sodium vapor lamp
used for public lighting. If the light has a wavelength of
589 nm, what is the frequency of this radiation? What
is its energy?
2. What type of radiation is involved if the wavelength
is 10 m? What type of radiation is involved if the
frequency is 5 x 1016 Hz? What wavelength is this?
3. Calculate the ionization energy of an Iridium atom,
given that radiation of wavelength 76.2 nm produces
electrons with a speed of 2980 km/s.
Workshop on Photoelectric Effect
1. Calculate the ionization energy of a rubidium atom,
given that radiation of wavelength 58.4 nm produces
electrons with a speed of 2450 km/s.
2. The energy required for the ionization of a certain
atom is 3.44 x 10-18 J. The absorption of a photon of
unknown wavelength ionizes the atom and ejects an
electron with velocity 1.03 x 108 m/s. Calculate the
wavelength of the incident of radiation.
3. The ionization energy of gold is 890.1 kJ/mol. Is light
with a wavelength of 225 nm capable of ionizing a gold
atom in the gas phase?
de Broglie Relation and the Wave-Particle Duality of Matter
If electromagnetic radiation has a dual character,
could it be that matter, which has been regarded as
consisting of particles, also have wavelike properties? On
the basis of theoretical observations, Louis de Broglie
suggested that all particles should be regarded as having
wavelike properties. Therefore, it was proposed that:
= h/p = h/mv
where = wavelength, h = Planck’s constant, p = linear
momentum (in units of kg m/s), m = mass (kg), and v =
velocity (m/s). BE CAREFUL: v is velocity and NOT
frequency (). Beginning chemistry students often confuse
these symbols!
Example: If an electron travels at a velocity of 1.000 x 107 m/s and
has a mass of 9.109 x 10-28 g, what is its wavelength?
Heisenberg Uncertainty Principle
Heisenberg determined that there is a fundamental
limitation to just how precisely one can know both the
position and the momentum of a particle at a given time.
That is,
x p > h/4
where x = the location of particle, p = linear momentum,
and h = Planck’s constant. This relationship means that
the more precisely we know a particle’s motion, the less
precisely we can know its momentum, and vice versa. The
uncertainty principle implies that we cannot know the
exact path of the electron as it moves around the nucleus.
It is therefore not appropriate to assume that the electron
is moving around the nucleus in a well-defined orbit.
Example: An electron with a mass of 9.109 x 10-31 kg is known to
have an uncertainty of 1 pm in its position. Determine the
uncertainty in its speed.
NIELS BOHR (1885-1962)
A MODEL for THE HYDROGEN ATOM
Bohr built upon Planck’s & Einstein’s ideas about quantized energy.
States:
1. The hydrogen atom has only specific allowable energy levels
(quantized).
2. The atom does not radiate energy while within an energy level.
3. Electrons transition to different energy levels only by absorbing
or emitting a photon whose energy equals the difference in energy
between the levels.
Ephoton = Efinal - Einitial = h
Limitations:
1. Failed to predict the spectrum for other atoms. (does not take into
account additional nucleus-electron attractions and electronelectron repulsion)
2. Electrons do not move in “orbits”
LINE SPECTRUM
Bohr’s model was an attempt to explain how light was emitted
when an element was vaporized and then thermally or electrically
excited (the flame test or neon sign). Through experimentation it
was discovered that each element has a unique line spectrum with
specific wavelengths that can be used to identify it.
RYDBERG EQUATION:
An empirical equation used to predict the position and
wavelength of the lines in a given series in a specific
region of the EM spectrum.
1 = R (1 - 1)
n12 n22
R = Rydberg constant 1.096776 x 107 m-1
BOHR’S MODEL & RYDBERG Combined 1913
RH = 2.179 x 10-18 J - Rydberg constant
The electron in the atom occupies specific energy levels (h, 2h,
3h, etc.) This is called QUANTIZATION
E = RH
n2
The electron may undergo a transition from one energy level to
another. Remember that the energy of emitted photon=hv=Ei - Ef
hv = RH (1 - 1)
ni2 nf2
1 = RH (1 - 1)
hc ni2 nf2
These equations can be used to determine the or v of the
hydrogen atom.
ENERGY STATES OF THE HYDROGEN ATOM
When an electron, in its ground state, absorbs energy from
a photon (called absorption), that electron is promoted to a
higher energy level (called the excited state). Emission is when
an electron in an excited state loses energy and returns to a lower
energy level. There is a direct relationship between emission &
absorption.
E = -2.18 x 10-18 J (Z2/n2)
E : the energy of the atom (derived from classical physics)
Z : the charge of the nucleus
n : the energy level
To find the energy difference between any two levels and predict
the spectral lines for the hydrogen atom:
E = h = hc
E = Ef - Ei = -2.18 x 10-18 J (nf-2 - ni-2)
R = -2.18 x 10-18 J/(hc)
The problem with classical physics of the time was that an electron
orbiting a nucleus would lose energy & eventually collapse into the
nucleus. In Bohr’s model, an electron can travel around a nucleus
without radiating energy. Furthermore, an electron in a given orbit has
a certain definite amount of energy. The only way an electron can lose
energy is by dropping from one energy level to a lower one. When this
happens, the atom emits a photon of radiation corresponding to the
difference in energy levels. However, electrons in higher levels cannot
drop to a lower level if that level is filled. Because no electrons can
move to a lower level, none of them can lose energy. The atom is
energetically stable and is said to be in its ground state.
Atoms can absorb energy from an outside source (such as heat from a
flame or electrical energy from a source of voltage), causing one or
more of the electrons within the atom to move to higher energy levels.
When electrons are moved to these higher levels, the atom is said to be
in an excited state. However, the atom is energetically unstable, so it
will not remain in an excited state for a long period of time. Eventually,
electrons return to lower levels. As they do so, energy is given off in
the form of quanta.
If ni nf - energy is emitted
________es
________gs
the electron jumps from a higher
energy level down to a lower energy
level
If ni nf - energy is absorbed
________es
________gs
the electron jumps from a lower
energy level to a higher one.
Example: Calculate the wavelength of light that corresponds to the
transition of the electron from n = 4 to n = 2 state of the
hydrogen atom. Is the light emitted or absorbed? What color is it?
Bohr Model Revisited
Bohr proposed a model that included the idea that the
electron in a hydrogen atom moves around the nucleus only in
certain allowed circular orbits. Furthermore, Bohr concluded the
following as applied to the bright-line spectrum of hydrogen:
1. Hydrogen atoms exist in only specified energy states given by the
Rydberg energy equation:
E = -2.178 x 10-18 J (Z2/nfinal2 - Z2/ninitial2)
where E = Energy (J), Z = atomic number, and n = orbital level.
2. Hydrogen atoms can absorb only certain amounts of energy, and
no others.
3. When excited hydrogen atoms lose energy, they lose only certain
amounts of energy, emitted as photons.
4. The different photons given off by hydrogen atoms produce the
color lines seen in the bright-line spectrum of hydrogen. The greater
the energy lost by the atom, the greater the energy of the photon.
Workshop on Bohr’s Model
1. Determine the energy of the line in the spectrum of
hydrogen that represents the movement of an electron
from a Bohr orbit with n = 6 to n = 4.
2. Determine the wavelength of light that must be
absorbed by a hydrogen atom in its ground state to
excite it to the n = 2 orbit.
NOTE:
At first, Bohr’s model appeared to be very promising. The energy
levels calculated by Bohr closely agreed with the values obtained
from the hydrogen emission spectrum. However, when Bohr’s
model was applied to atoms other than hydrogen, it did not work
at all. It was therefore concluded that Bohr’s model is
fundamentally incorrect for atoms with more than one electron.
Schrödinger Equation
Erwin Schrödinger proposed the theory of quantum
mechanics which suggested that an electron (or any other
particle) exhibiting wavelike properties should be described
by a mathematical equation called a wavefunction (denoted
by the Greek letter psi, ). Specifically, he developed a
mathematical formalism to describe the hydrogen atom as
a wave. This equation (which involves differential
calculus!) gives the probability of finding an electron at
some point in a three-dimensional space at any given
instant but offers no information about the path the
electron follows (recall Heisenberg). The region in space where
there is a probability of finding an electron is known as an
orbital, & in the Schrödinger equation, the wavefunction is
used to calculate the probability of finding an electron in
space.
QUANTUM MECHANICS
Erwin Schrodinger (1887 - 1961)
Schrodinger formulated the theory of wave
mechanics: a description of the behavior of the tiny
particles that make up matter in terms of waves. His wave
equation describes the behavior of electrons in atoms.
HY=EY
Y is the wave function/atomic orbital: a mathematical
description of the motion of the electron’s matter-wave in
terms of position & time.
H is the Hamiltonian operator
E is the energy of the atom
d2 Y/dx2 + d2Y/dy2 + d2Y/dz2 + (82me/h2)[E-V(x,y,z)] Y(x,y,z)
Y2 is the probability of an electron being within a volume.
Quantum Mechanics
In quantum mechanics, the electrons occupy
specific energy levels (as in Bohr's model) but
they also exist within specific probability
volumes
called
orbitals
with
specific
orientations in space. The electrons within
each orbital has a distinct spin.
n = The principle quantum number
Describes the possible energy levels and
pictorially it describes the orbital size.
n = 1, 2, 3…. where an orbital with the value of 2 is
larger than an orbital with the value of 1.
E n -2.1810
-18
1
J 2 for an electron in H 1s
n
2s
Principal Energy Levels in Hydrogen
40
Quantum Mechanics
l = angular momentum quantum number
Describes the "shape" of the orbital and can have values
from 0 to n - 1 for each n.
l = (n-1) to 0
(2 l + 1 subshells)
orbital designation :
s
p
d
f
Too hard to draw
see text
shape :
ml = magnetic quantum number
Related to the orientation of an orbital in space relative to
the other orbitals with the same l quantum numbers. It
can have values between l and - l .
ms = spin quantum number
An electron has either +1/2 or -1/2 spin values; sometimes
referred to as spin up and spin down.
Why are Atoms Spherical?
45
Energy Shells and Subshells
46
Quantum Mechanics and the Periodic Table
1. Principle Quantum Number (n): specifies the energy level of an
electron and labels the shell of an atom. Represented by the period
number.
2. Angular Momentum Quantum Number (l): specifies the subshell
of a given shell in an atom and determines the shape of the orbitals
in the subshell.
Period
1
2
3
4
5
6
7
p
s
d
f
3. Magnetic Quantum Number (ml): identifies the individual
orbitals of a subshell of an atom and determines the orientation
in space
4a. Spin Quantum Number (ms): distinguishes the two spin states
of an electron
n
1
2
3
4
l
Orbital Designation
ml
# of orbital
4b. Pauli Exclusion Principle: No two electrons can have the same
four sets of identical quantum numbers. Moreover, when two
electrons (and NO MORE THAN TWO!) occupy the same given
orbital, their spins must be different. This is due to the spin
number.
Lecture Questions on Quantum Mechanics
1. Determine the quantum numbers associated
with the following energy levels:
a) n=2
b) n= 3
n=5
2. Determine the sublevel names & quantum
numbers:
a) n = 3, l = 2
b) n = 4, l = 2
c) n = 2, l = 2
3. What is the maximum number of electrons in
an atom that can have these quantum numbers?
A. n = 3
B. n = 2, l = 0, ml = 0
C. n = 5, ms = +½
D. n = 2, l = 1
Workshop on quantum numbers
1. How many subshells are there for n = 6? How many total
orbitals are there in the shell with n = 6?
2. Write the subshell notation and the number of electrons that
can have the following quantum numbers if all the orbitals of that
subshell are filled:
A.
C.
n = 3; l = 2
n = 7; l = 1
B.
D.
n = 5; l = 0
n = 4; l = 3
3. How many electrons can have the following quantum numbers
in an atom:
A.
B.
C.
n = 3, l = 1
n = 5, l = 3, ml = -1
n = 2, l = 1, ml = 0
4. Which of the following sets of quantum numbers {n, l, ml, ms}
are allowed and which are not? For the sets of quantum numbers
that are incorrect, state what is wrong.
A.
B.
C.
{2, 2, -1, -½}
{6, 0, 0, +½}
{5, 4, +5, +½}








