Chemistry is a prerequisite course for a wide range of college
advertisement

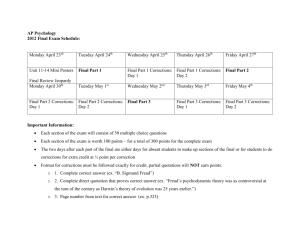
Chemistry Introduction Chemistry is a prerequisite course for a wide range of college majors for two reasons. One, the content knowledge is used in a variety of fields including medicine, engineering, biology, geology and physics. Two, the problem solving skills developed in chemistry have a wide range of applications in nearly all fields of study. Following are the 11 units and a brief list of topics. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. Unit Structure of Matter Periodic Table Chemical Bonding States of Matter Stoichiometry—General Stoichiometry—Aqueous Electrochemistry Thermodynamics Kinetics Equilibrium—General Equilibrium—Aqueous Major Topics Measurement, Atomic Nature, Radioactivity, Bohr Model Quantum Model, Electron Structure, Periodic Properties Bonding, Molecular Geometry, Organic Chemistry Gases, Liquids, Solids, Solubility, Colligative Properties Balanced Equations, Gravimetric Analysis Volumetric Analysis, Precipitation Reactions, Acid-Base Reactions, Oxidation-Reduction Reactions Oxidation-Reduction, Standard Potential, Voltaic Cell, Electrolytic Cell Enthalpy and Entropy, Thermodynamic Analysis Reaction Rate, Reaction Mechanism Equilibrium State, Le Chatelier's Principle Acid-Base Equilibrium, Buffer Systems, pH profile, Solubility Equilibrium, Solubility Factors Supplies and Materials 1. 2. You will need: a. 3-ring binder with notebook paper b. highlighter pen c. scientific calculator d. ruler e. $10.00 lab fee Textbook: Chemistry, The Central Science; Brown, LeMay and Bursten Class Process New material is presented in lecture format. You are provided with a work packet, which includes lecture notes, experiments, practice problems, practice multiple choice test questions and practice free response test questions. The work packet is also available online. Lectures and experiments are completed in class (although you may need to return during lunch or 7 th period to complete the lab). Practice problems, practice multiple choice and practice free response are done as homework. YOU ARE RESPONSIBLE FOR ALL WORK, INCLUDING LABS, EVEN FOR DAYS THAT YOU ARE ABSENT. After completing a unit, you take a two-part test. You turn in your work packet (10 points), take a free response test (10 points) with one of your lab partners (on rotation), then take a multiple choice test (30 points) by yourself. If you are absent for a test, you are expected to make up it up (by yourself) as soon as you can, preferably the next day at lunch or 7 th period. Honors students may use a 3 x 5 index card on the multiple choice portion of the test. In addition they will receive five extra points. Corrected tests are returned the next class period. You can earn 20% of the points missed on the test by doing corrections. Test corrections include explanations (or corrected calculations) for each missed multiple choice and free response (similar to what you do for practice), indication whether you missed the problem due to careless error or didn't know the answer, and a brief description of what you can do next time to improve your score. Test corrections are due the next period after receiving the corrected test. For students who do corrections for EVERY test in the semester will receive bonus points equal to 1 % of the total points that semester. The first semester content is completed before winter break. The remaining time is set aside for reviewing and preparing for the semester finals. The process starts during break, when you will review units 1-7, complete practice multiple choice and free response tests and do test corrections (extra credit equal to the test correction points for your finals will be earned by turning in the completed tests and test corrections). For the two weeks after break you will take a series of practice multiple choice and free response tests and do test corrections (10 points). The final includes a lab team portion (50 points), a partner free response portion (40 points) and a solo multiple choice portion (50 points). The total for the semester is 500 un-weighted points. The second semester content is completed before spring break, and like the first semester, the remaining time is set aside for review and preparation for the AP exam and the class finals. During spring break you will review units 1-11, complete practice multiple choice and free response tests and do test corrections (extra credit equal to the test correction points for your finals will be earned by turning in the completed tests and test corrections). For the four weeks after break you will listen to a quick review, take a series of practice multiple choice and free response tests and do test corrections for all missed questions (10 points). In addition, a mock multiple choice test will be given. If you get at least 40 out of 50 correct, you will receive an automatic 50 points on the multiple choice final. The final includes a lab team portion (50 points), a partner free response portion (40 points) and a multiple choice portion (50 points). After the final you complete a lab project, Qualitative Analysis, where your lab team performs a series of separation and identification steps in order to identify the contents of an unknown (50 points). The total for the semester is 400 un-weighted points.