Chapter 1: Fundamental Concepts
advertisement
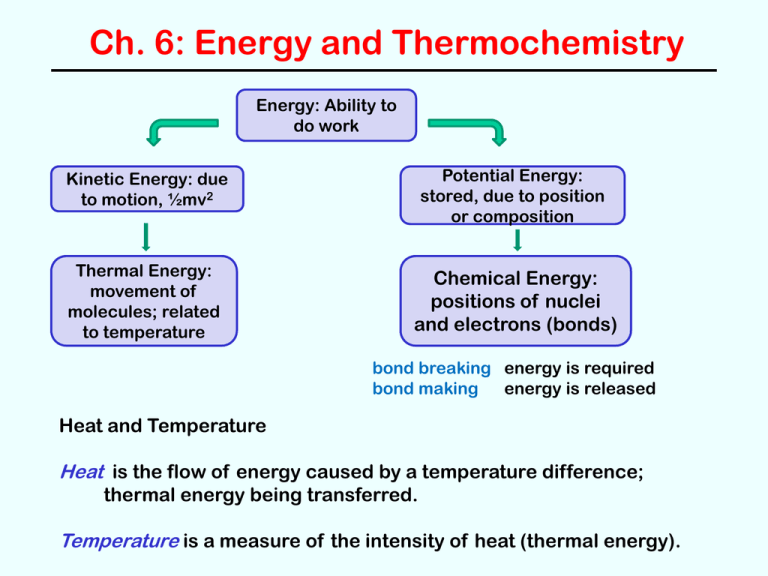
Ch. 6: Energy and Thermochemistry
Energy: Ability to
do work
Kinetic Energy: due
to motion, ½mv2
Potential Energy:
stored, due to position
or composition
Thermal Energy:
movement of
molecules; related
to temperature
Chemical Energy:
positions of nuclei
and electrons (bonds)
bond breaking energy is required
bond making
energy is released
Heat and Temperature
Heat is the flow of energy caused by a temperature difference;
thermal energy being transferred.
Temperature is a measure of the intensity of heat (thermal energy).
Internal Energy
-E = internal energy = KE + PE
-Energy Changes
When reactions occur there is an energy change DE:
DE = Efinal - Einitial
Efinal = energy of products
Einitial = energy of reactants
reactants
E
products
DE = q + w
where q = heat
w = work
sign convention: positive if system gains energy
Measuring Thermal Energy
1. Units of Energy (see Table 6.1)
kg•m2/s2
SI: 1 J = 1
1 cal = 4.184 joule
1 kcal = 1 “Cal”
memorize!
(½mv2)
(exactly)
2. Heat Capacity, C
--amount of heat required to raise the temp of substance by 1 °C
units: energy/temp (J/°C or kJ/°C or cal/°C)
quantity of heat = (heat capacity) x Dt
3. Specific Heat, Cs
Heat Capacity of specified mass of substance (1 gram)
units: usually J/g °C or cal/g °C
e.g. specific heat of water = 1.00 cal/g °C = 4.18 J/g °C
quantity of heat = (specific heat) x mass x Dt
4. Molar Heat Capacity:
– Heat Capacity per mole of a substance
– e.g. molar heat capacity of water = 18.0 cal/mole °C
= J/g °C x g x °C
Example Problems
Problem
The temp of 250 g H2O (3 sig fig) is raised from 25.0 °C to 30.0 °C.
How much heat energy is required?
Dt = 30.0 - 25.0 = 5.0 °C
Amount of heat = (1.00 cal/g °C) x (250 g) x (5.0 °C)
= 1,250 cal = 1.25 kcal
= 1,250 cal x 4.184 J/cal
= 5320 J = 5.3 kJ
Problem
Identify each energy change as primarily heat or work, and determine
whether Esys is positive or negative.
a. One billiard ball (the system) hits another one, and stops rolling.
b. A book (the system) is dropped on the floor
c. A father pushes his daughter on the swing (the daughter & swing
are the system)
a. work, negative
b. work, negative
c. work, positive
Thermal Energy Transfer
Thermal energy flows from matter at higher temperature to matter at
lower temp, until thermal equilibrium.
qA = –qB
Example Problem
A 3.35 g iron rod, initially at 22.7 °C, is submerged into an unknown
mass of H2O at 63.2 °C, in an insulated container. The final temp of
the mixture is 59.5 °C. What is the mass of the water? (Cs iron = 0.449
J/g∙ °C, Cs water = 4.18 J/g ∙ °C)
m x CS,Fe x DTFe = –m x CS,H2O x DTH2O
DTFe = 59.5 – 22.7 = 36.8 °C for Fe
DTH2O = 59.5 – 63.2 = –3.7 °C for H2O
(3.35)(0.449)(36.8) = –m(4.18)(– 3.7)
m = (3.35)(0.449)(36.8)/(4.18)(3.7) = 3.6 g
Internal Energy and Enthalpy
E = internal energy = KE + PE
H = enthalpy = E + PV
DE = total energy change
DH = total heat change
(when P is constant)
(see book for derivation)
Bomb Calorimeter
constant V; measures DE
Coffee-Cup Calorimeter
constant P; measures DH
Internal energy and enthalpy are state functions. The energy change
(DE) and heat change (DH) of a reaction depend only on the initial and
final states of the system -- not on the specific pathway.
Enthalpy Changes in Chemical Reactions
exothermic reaction
endothermic reaction
heat is a product of the reaction
-- gives off heat to the surroundings
-- system warms up
heat is essentially a reactant
--absorbs heat from the surroundings
-- system cools off
Enthalpy (H) -- “Heat Content”
– The total energy of a chemical system at constant pressure
DH = Hproducts - Hreactants
endothermic reaction
exothermic reaction
DH > 0 (positive) -- heat is absorbed
DH < 0 (negative) -- heat is released
Standard Heat of Reaction (DH°)
DH = the value of DH for a reaction as written.
DH° = the value of DH for a reaction:
– Under standard conditions (temp = 25 °C, pressure = 1 atm)
– With actual # moles specified by coefficients in balanced eqn
e.g. reaction for the combustion of ethylene:
C2H4(g) + 3 O2(g) --> 2 CO2(g) + 2 H2O(l)
DH° = -1411 kJ (very exothermic)
i.e. 1411 kJ of heat energy are released in the reaction of 1 mole of
C2H4 with 3 moles of O2
Problem
If 10.0 g of C2H4 are burned, how much heat is produced?
(10.0 g C2H4) x (1 mole C2H4/28.0 g C2H4) x (1411 kJ/mole C2H4)
= 504 kJ
Manipulating Thermochemical Equations
If reaction is reversed, change sign of DH°.
If reaction is multiplied or divided by a factor, apply same
factor to DH°.
DH° for overall reaction = sum of DH° values for individual
reactions.
Problem
Given the following thermochemical reactions:
(eq 1) C2H4(g) + 3 O2(g) --> 2 CO2(g) + 2 H2O(l) DH° = -1411 kJ
(eq 2) C2H5OH(l) + 3 O2(g) --> 2 CO2(g) + 3 H2O(l) DH° = -1367 kJ
Calculate DH° for the following reaction:
C2H4(g) + H2O(l) --> C2H5OH(l)
Example Problem, cont.
Reverse 2nd reaction to put C2H5OH on product side then
rewrite 1st equation and add them together.
(eq 2)
2 CO2(g) + 3 H2O(l) --> C2H5OH(l) + 3 O2(g)
DH° = + 1367 kJ (note the sign change!!!)
(eq 1)
C2H4(g) + 3 O2(g) --> 2 CO2(g) + 2 H2O(l)
DH° = -1411 kJ
Net:
C2H4(g) + H2O(l) --> C2H5OH(l)
{note: 3 O2, 2 CO2, and 2 H2O cancel out}
DH° = DH°1 + DH°2 = 1367 + (-1411) = -44 kJ
Standard Heat of Formation
•
Standard heat (enthalpy) of formation of a substance:
DH°f =
DH° for the formation of one mole of substance from
the elements in their standard states
a “formation” reaction
H2(g) + 1/2 O2(g) --> H2O(l)
DH°f (liq water) = -286 kJ/mole
DH°f is a property of a substance -- see text for examples
•
Practice writing formation reactions -- e.g. Na2SO4
2 Na(s) + 2 O2(g) + S(s) --> Na2SO4(s)
DH°f = -1385 kJ/mole
Hess’ Law of Heat Summation
• Calculate DH° for a reaction from tabulated DH°f values
DH° = S DH°f (products) - S DH°f (reactants)
Problem
Determine DH° for the following reaction from DH°f values.
2 H2O(l) + CaSO4(s) --> CaSO4•2H2O(s)
DH° = DH°f[CaSO4•2H2O(s)] - {DH°f[CaSO4(s)] + 2 DH°f[H2O(l)]}*
= (-2021.1) - {(-1432.7) + 2(-285.9)}
= -16.6 kJ
{*units: e.g., (2 moles) x (285.9 kJ/mole) = kJ}
Summary
two ways to get DH° for a reaction:
– By manipulating 2 or more given equations, then adding their
DH°’s
– From tabulated DH°f values using Hess’ Law
Sample Problems
• Write a balanced chemical equation that represents the
formation reaction for (NH4)3BO3.
• Given the following thermochemical equations, calculate
the standard heat of formation (DH°f) of Mg3N2(s) in kJ/mole.
Mg3N2(s) + 3 H2(g) --> 3 Mg(s) + 2 NH3(g) DH° = 371 kJ
1/2 N2(g) + 3/2 H2(g) --> NH3(g)
DH° = -46 kJ
Sample Problems
• Write a balanced chemical equation that represents the
formation reaction for (NH4)3BO3.
3/2 N2(g) + 6 H2(g) + 3/2 O2(g) + B(s) --> (NH4)3BO3(s)
• Given the following thermochemical equations, calculate
the standard heat of formation (DH°f) of Mg3N2(s) in kJ/mole.
Mg3N2(s) + 3 H2(g) --> 3 Mg(s) + 2 NH3(g) DH° = 371 kJ
1/2 N2(g) + 3/2 H2(g) --> NH3(g)
Answer:
3 Mg(s) + 2 NH3(g) --> Mg3N2(s) + 3 H2(g)
DH° = -371 kJ
N2(g) + 3 H2(g) --> 2 NH3(g)
DH°f = 2(-46 kJ)
3 Mg(s) + N2(g) --> Mg3N2(s) [the formation rxn for Mg3N2]
DH° = -371 + 2(-46) = -463 kJ
DH°f for Mg3N2(s) = -463 kJ/mole
Sample Problem
• The specific heat of copper is 0.387 J/g °C. The molar heat
of fusion of water is 6.0 kJ/mole. If a copper rod weighing
225 g is heated to 80 °C and then immersed in 100 g of ice at
0 °C, how many grams of ice will melt?
Sample Problem
• The specific heat of copper is 0.387 J/g °C. The molar heat
of fusion of water is 6.0 kJ/mole. If a copper rod weighing
225 g is heated to 80 °C and then immersed in 100 g of ice at
0 °C, how many grams of ice will melt?
Answer:
Heat lost by Cu = heat gained by ice
(0.387 J/g °C)(225 g)(80 °C) = 6966 J = 6.966 kJ
(6.966 kJ)(1 mole ice/6.0 kJ)(18.0 g ice/mole ice) = 21 g ice







