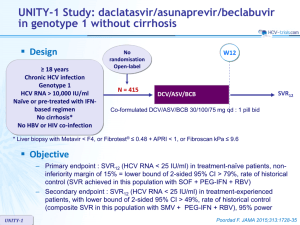
this slide kit
advertisement
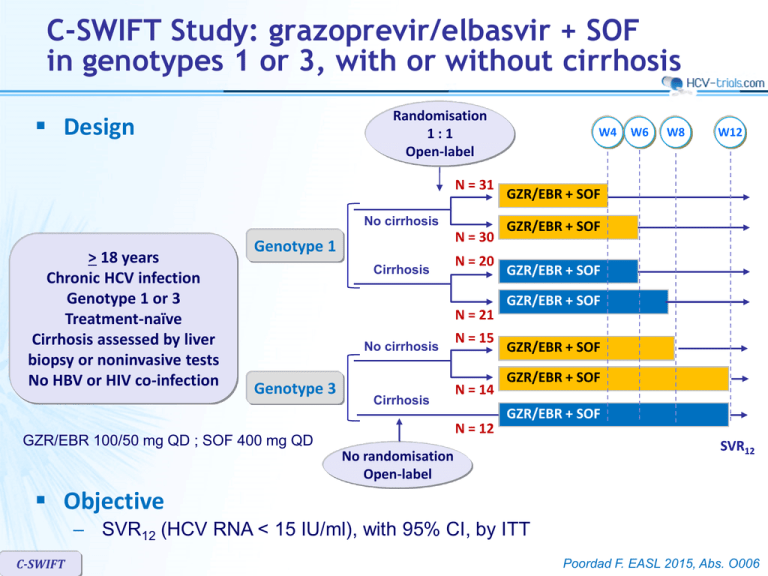
C-SWIFT Study: grazoprevir/elbasvir + SOF in genotypes 1 or 3, with or without cirrhosis Randomisation 1:1 Open-label Design N = 31 No cirrhosis > 18 years Chronic HCV infection Genotype 1 or 3 Treatment-naïve Cirrhosis assessed by liver biopsy or noninvasive tests No HBV or HIV co-infection N = 30 Genotype 1 Cirrhosis N = 20 N = 21 No cirrhosis Genotype 3 Cirrhosis N = 15 N = 14 N = 12 GZR/EBR 100/50 mg QD ; SOF 400 mg QD W4 W6 W8 W12 GZR/EBR + SOF GZR/EBR + SOF GZR/EBR + SOF GZR/EBR + SOF GZR/EBR + SOF GZR/EBR + SOF GZR/EBR + SOF No randomisation Open-label SVR12 Objective – SVR12 (HCV RNA < 15 IU/ml), with 95% CI, by ITT C-SWIFT Poordad F. EASL 2015, Abs. O006 C-SWIFT Study: grazoprevir/elbasvir + SOF in genotypes 1 or 3, with or without cirrhosis Baseline characteristics Genotype 1 No cirrhosis Genotype 3 Cirrhosis No cirrhosis Cirrhosis 4 weeks N = 31 6 weeks N = 30 6 weeks N = 20 8 weeks N = 21 8 weeks N = 15 12 weeks N = 14 12 weeks N = 12 52 51 56 57 51 42 55 Female 35% 37% 35% 38% 27% 43% 17% Race, white 97% 93% 100% 100% 95% 100% 100% IL28B CC 36% 27% 30% 24% 40% 21% 50% Genotype 1a 1b 84% 16% 87% 13% 80% 20% 76% 24% - - - 0 0 20 (100) 21 (100) 0 0 12 (100) 3.69 3.09 1.66 2.37 3.29 2.57 2.26 Mean age, years Cirrhosis, N (%) HCV RNA x 106 IU/ml, mean C-SWIFT Poordad F. EASL 2015, Abs. O006 C-SWIFT Study: grazoprevir/elbasvir + SOF in genotypes 1 or 3, with or without cirrhosis SVR12 (HCV RNA < 15 IU/ml), mITT, Genotype 1 Non-cirrhotic Cirrhotic % 100 87 80 94 80 60 40 33 20 0 Breakthrough Relapse Non virologic failure 30 30 20 20 4 weeks 0 20 1 6 weeks 0 4 0 6 weeks 0 4 0 8 weeks 0 1 3 Poordad F. EASL 2015, Abs. O006 C-SWIFT Study: grazoprevir/elbasvir + SOF in genotypes 1 or 3, with or without cirrhosis SVR12 (HCV RNA < 15 IU/ml), mITT, Genotype 3 % 100 93 Non-cirrhotic 100 Cirrhotic 91 80 60 40 20 0 Breakthrough Relapse Early discontinuation C-SWIFT 15 14 11 8 weeks 0 1 0 12 weeks 0 0 0 12 weeks 0 1 1 Poordad F. EASL 2015, Abs. O006 C-SWIFT Study: grazoprevir/elbasvir + SOF in genotypes 1 or 3, with or without cirrhosis SVR12 (HCV RNA < 15 IU/ml), per-protocol, Genotype 3, by subgroups % 100 95 100 97 85 91 100 93 96 28 12 14 26 Male Female CC Non-CC 93 80 60 40 20 0 40 All patients 27 <2M IU/ml 13 >2M IU/ml 29 No cirrhosis Cirrhosis Baseline HCV RNA C-SWIFT 11 Gender IL28B genotype Poordad F. EASL 2015, Abs. O006 C-SWIFT Study: grazoprevir/elbasvir + SOF in genotypes 1 or 3, with or without cirrhosis Resistance analysis at failure NS3 RAV NS5A RAV Genotype 1 (29 relapses) NS5B RAV 56 28/29 (97%) 18/30 (60%) 30/30 (100%) 0 1 (3%) 0 1 (3%) 9 (30%) 4/9 in 4W arm 0 0 2 (7%) 0 At baseline Q168Q/R 0 0 At relapse Q168R Y93H 0 No resistance-associated variants Pre-existing baseline RAVs only RAVS detected at failure RAVs at failure in addition of baseline RAVs Genotype 3 (2 relapses) C-SWIFT Poordad F. EASL 2015, Abs. O006 C-SWIFT Study: grazoprevir/elbasvir + SOF in genotypes 1 or 3, with or without cirrhosis Adverse events, N (%) Genotype 1 All patients Genotype 3 Non cirrhotic 4 & 6 weeks N = 61 Cirrhotic 6 & 8 weeks N = 41 Non cirrhotic 8 & 12 weeks N = 29 Cirrhotic 12 weeks N = 12 Discontinuation due to AE 1 (1) 0 1 (2) 0 0 Serious adverse event 2 (2) 0 2 (5) 0 0 0 0 0 0 0 4 (4) 1 (2) 2 (7) 1 (3) 1 (8) 2 (2) 2 (2) 2 (3) 2 (3) 0 0 1 (8) 1 (2) 1 (3) 1 (8) 0 0 0 Death Most common AEs Headache Fatigue Nausea Hemoglobin < 10 g/dl 0 Total bilirubin > 5 x baseline 0 0 0 0 0 ALT/AST > 5 x ULN 0 0 0 0 0 C-SWIFT Poordad F. EASL 2015, Abs. O006 C-SWIFT Study: grazoprevir/elbasvir + SOF in genotypes 1 or 3, with or without cirrhosis Summary – Grazoprevir/elbasvir + sofosbuvir was able to shorten treatment duration to 8 weeks or less among cirrhotic and non-cirrhotic HCV genotype 1 infected patients – Genotype 3 patients achieved high SVR12 rates with 8-12 weeks of therapy, including patients with cirrhosis – All virologic failures were due to relapse – Patients relapsed most commonly with either wild-type virus or with RAVs already present at baseline – GZR/EBR + SOF was generally safe and well tolerated C-SWIFT Poordad F. EASL 2015, Abs. O006