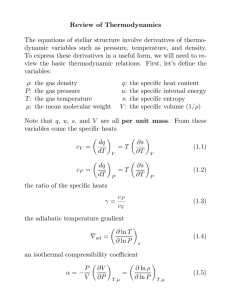
Adiabatic processes on a thermodynamic chart.
advertisement
Adiabatic processes on a thermodynamic chart. Atms Sc 4310 / 7310 Lab 2 Anthony R. Lupo Adiabatic processes on a thermodynamic chart. 1st law of thermodynamics in the form (imperfect form) dq dT dP Q cp dt dt dt dq source sin k dt is a statement of the Conservation of energy (heat in = heat out)! Adiabatic processes on a thermodynamic chart. Left hand side (represents?): Diabatic heating, which includes Latent Heat Release, Sensible Heating, and Radiational Heating. Adiabatic processes on a thermodynamic chart. Right hand side: Term 1: Internal Energy (due to Temperature) Term 2: “pressure work” term or work done by pressure by the environment on a parcel. (expansion – heat added to system diabatically contraction - heat removed diabatically) Adiabatic processes on a thermodynamic chart. Adiabatic process dq/dt = 0 (no work done)!! sources sin ks Thus, the internal energy equals the pressure work term. Also, now there are no diabatics so; dT dp cp dt dt Adiabatic processes on a thermodynamic chart. Expansion (lower pressure) cooling work done on air parcel and energy lost to environment Contraction (higher pressure) warming work done by environment and energy gained at expense of environment Adiabatic processes on a thermodynamic chart. Constant Potential Temperature Adiabatic system or flow follows lines of potential temperature. Why? Let’s derive relationship for potential temperature Adiabatic processes on a thermodynamic chart. Derive…..start with 1st law 0 cp dT dP dt dt invoke T cp To " the dT dt snake" P dP dt Po then RT P so T 1 dT R T dt cp To P 1 dP P dt Po Adiabatic processes on a thermodynamic chart. And…. ln T ln T To R P ln P Po cp T R P ln To cp Po call To call R k cp T P Po Po P Adiabatic processes on a thermodynamic chart. Some adiabatic processes Adiabatic motion on the thermodynamic diagram: no work done, potential temperature is constant. Use adiabatic motion as a first estimate of maximum temperature Adiabatic processes on a thermodynamic chart. LCL – lifting condensation level (air lifted adiabatically until saturation!) LFC – level of free convection: rising parcel becomes warmer than environment, rises under it’s own power, or due to bouyant forces. CCL – convective condensation level: Raise parcel along. Environmental sounding until saturation (intersect mixrat. And sounding) Adiabatic processes on a thermodynamic chart. Convective temperature -- take parcel down dry adiabatically to surface, that’s the temp we must get to to get convection. Equilibruim level – where parcel becomes neutrally bouyant again. Adiabatic processes on a thermodynamic chart. Convective available potential energy: + value: parcels warmer than environment which gains energy from the air parcels - value: parcels cooler than environment which must do work to lift parcels and loses energy. Adiabatic processes on a thermodynamic chart. Other buoyancy related indicies: Lifted Index Showalter Index Energy Index Adiabatic processes on a thermodynamic chart. Questions? Comments? Criticisms? Adiabatic processes on a thermodynamic chart. The end!