File
advertisement

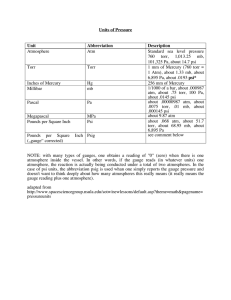
Gas Laws - Additional Practice Name: ______________________________________________ Hour: __________________ 1. Convert the following pressures into atmospheres. a. 105.2 kPa b. 75.2 cm Hg c. 752 mm Hg d. 767 torr 2. If the pressure exerted on the gas in a weather balloon decreases from 1.01 atm to 0.562 atm as it rises, by what factor will the volume of the gas in the balloon increase as it rises? 3. What will the volume of the sample become if 459 mL of an ideal gas at 27 °C Show all work – Please be neat. 4. Complete the Table. (show ALL work.) Torr Atm Pascals mmHg 440 Torr 130,550 Pa 2.82 atm 942 mmHg 5. If 5.00 L of an ideal gas is cooled from 24 °C to -272 °C, what will the volume of the gas become? Show all work – Please be neat. 6. Determine the pressure in a 125-L tank containing 56.2 kg of oxygen gas (how many moles of O2, 32 grams per mol) at 21 °C. 7. What will be the new volume if 125 mL of He gas at 100. °C and 0.981 atm is cooled to 25 °C and the pressure is increased to 1.15 atm? 8. A 1.04-L sample of gas at 759 mm Hg pressure is expanded until its volume is 2.24 L. What is the pressure in the expanded gas sample (at constant temperature)? Show all work – Please be neat.