9. (StI-BHEM) BIOCHEMISTRY - Medicinski Fakultet Univerzitet Novi
advertisement

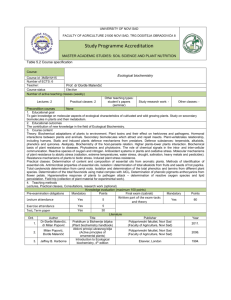
9. (StI-BHEM) BIOCHEMISTRY STUDY PROGRAMME DEPARTMENT Integrated studies of dentistry Departmen for biochemistry NAME OF SUBJECT BIOCHEMISTRY Compulsory STATUS OF THE SUBJECT Condition none Winter term (No.of the Summer term (No.of the No.of No.of Year of lessons per week lessons per week) No.of tests seminars seminars studies seminara POINTS Lectures Exercises Lectures. Exercises first 3 3 2 7,0 Methods of conducting Lectures for bigger and smaller groups using multimedia. Testing. Practical knowledgeteaching conducting biochemical analysis and reading results. GOA L PURPOSE The goal of teaching biochemistry is to teach students to understand all biological processes in our body, to learn about basic methods used in clinical chemistry as well as diagnostical means. To enable future doctors to use these methods in right manner. Knowledg Knowing basic chemical constituents of human body, general metabolical ways, understanding the essence of many diseases and knowing specific biochemical processes of some organs and tissues. e Appropriate taking of biological material for biochemical analysis. Assessing reliability of some biochemical methods and their use for diagnosis. Using results of biochemical analysis for diagnosis. Skills Examining metabolism the most important content of organism based on measures on biological samples.proving basic laws of biochemistry based on laboratory methods. CONTENT OF THE SUBJECT:: Theoretical teaching – methodical units 1. Introduction to biochemistry. 2. Water as biological solvent. 3. Aminoacids. Peptides.. 4. Proteins – structure, qualities, classification. Hemoproteiesi – hemoglobin, mioglobin i citohroms. 5. Nuclear acids, bases, DNA, genetic code, RNA. 6. Phospolipides and biological membranes. 7. Enzymes-chemical nature,enzyme catalysis. Cinetics of enzyme reaction. Classification. Coenzymes and vitamins. 8. Chemical thermodynamics. Bioenergetics. Biological oxidation. Transformation of energy. ETS,ATP. 9. Metabolical ways. Digesting. Glicolysis. Krebs's cycle of lemon acid. 10. Digesting of proteins and absorption of aminoacids. 11. Digesting and absorption of lipids. Metabolism of lipoproteins. 12. Beta oxidation of fatty acids. Regulation of the metabolism. 13. Synthesis of DNA-replication. Synthesis of RNA-transcription. Synthesis of proteins. 14. Water and electrolytes. 15. Calcium and its importance. 16. Parathormon, D-hormone i kalcitonin. 17. Biochemistry of blood-blood plasma, coagulation of blood, biochemistry of eritrocites. 18. Biochemistry of connective tissue 19.Bone,dentin,cement and enamel. 20. Oral biochemistry. . 21. Hormones. Mechanisms of action of steroid hormones. Hormones tireoideje. Adrenaline and norepinephrine. Hormones of the pancreas. Corticosteroids. Male and female sexual hormones. Hormones and aden neurohipofize. Practical teaching – methodical units 1. 2. 3. 4. 5. 6. 7. 8. Introduction. The aim of exercises. Summary of teaching. Checking the reliability of biochemical methods Photometry. Principles of Lambert-Beer's law. Ekstinkcioni and molar extinction coefficient. Blind test, and the standard calibration curve. Kolorimetar and spectrophotometer. Application of Photometry. Checking the Lambert-Beer's law and determining the concentration bromtimol blue. Kolorimetric determining the concentration of inorganic phosphate. Kolorimetric determination of protein. Methods for determining protein. Determination of protein concentration in serum biureten reaction, Isolation, fibrinogene method. Polarimetrija. Determining a specific angle of turning the plane of polarized light and glucose concentration glukoze u urinu. Quantitative determination of urea Berthelot method. Qualitative analysis of bile color in the serum and urine. 9. 10. 11. 12. 13. 14. Quantitative determination of chloride in serum. Qualitative proving enzymes. Enzymatic hydrolysis of starch-alpha amylase activity. Principles of quantitative measurement of enzyme activity. Determination of alkaline phosphatase activity in function of time. Proof of the existence izoenzima alkaline phosphatase. Jonochanging chromatography of amino acids. 1. S. Marinkov, J. Borota: Medicinska biohemija, Radnički univerzitet ”Radivoj Ćirpanov” Novi Sad, 2006. 2. J. Borota i saradnici: Praktikum medicinske biohemije i hemije, Medicinski fakultet, READING Novi Sad, 2006. Additional 1. Z.Kovačević: Biohemija i molekularna biologija, Medicinski fakultet, Novi Sad, 2006. Evaluation of students' work – No.of points per individual activity Pre-exam obligations Final exam Total Lectures Exercises Test Seminar paper The rest Written Oral 100 18 35 16 31 Compulsor RECOMMANDED y List of teachers and assistents Associate Assistent Lecturer 5 1. 2. 3. 4. Prof dr Jela Borota Doc. dr Katica Bajin Katić Doc dr Karmen Stankov Doc dr Ljiljana Andrijević Prof. struk. PhD 3 5. 6. 7. 8. 9. Associate prof. Professor Scientist 1 Asst. mr Mirjana Milošević-Tošić Asst. dr Tatjana Ćebović Asst. mr Jelena Stojčević-Maletić Asst. mr Jasmina Katanić Asst. mr Jovica Oros Chief of department Prof. dr Jela Borota ensuring