L-7 PlantCell osmosis LAB
advertisement

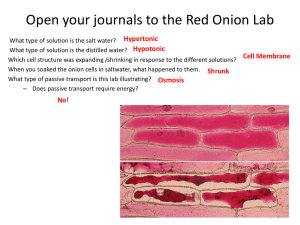
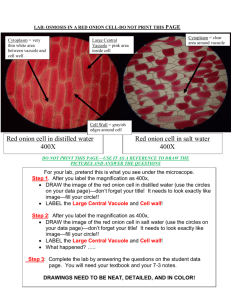
OSMOSIS IN A PLANT CELL LAB Cells lose or gain water due to the difference in concentrations between the cytoplasm and the solution surrounding the cell. The movement of water in and out of a cell is governed by the laws of diffusion: water flows from a region of higher water concentration to a region of lower concentration. When a cell is in a concentrated solution (like salt water), it will experience a loss of water. Saltwater contains a higher concentration of dissolved materials than the cell and therefore a lower concentration of water. Consequently, water will flow out of the cell from the region of higher water concentration to the region of lower water concentration. When a cell is in a dilute solution (like fresh water), it will experience a gain of water. Freshwater contains a lower concentration of dissolved materials than the cell and therefore a higher concentration of water. Consequently, water will flow into the cell from the region of higher water concentration to the region of lower water concentration. When a cell is in a balanced solution (like blood), it will experience neither a net gain or loss of water. A balanced solution contains an equal concentration of dissolved materials as the cell and therefore an equal concentration of water. Consequently, water will flow equally into and out of the cell and that will balance out. When a plant cell shrinks because of loss of water, we call that plasmolysis. Since plant cells have cell walls, the whole cell will not collapse when it first loses water. The cell wall will still remain as a rigid support. When a plant cell is put in water that has a lot of material dissolved in it, like salty water, the cytoplasm will shrink as it loses water through osmosis. Water diffuses out of the cell and into the more concentrated solution surrounding the cell. See Figure 3 below. During plasmolysis the cell membrane pulls away from the cell wall as the cytoplasm loses water. In this lab exercise, you will examine this process by observing the effects of a highly concentrated salt solution on plant cells. By the way, because animal cells do not have the support of a cell wall, they usually cannot survive treatment with a very concentrated solution, like the salty water we use in this lab. They shrivel too much to recover. Figure 3. IN FRESHWATER IN A BALANCED SOLUTION IN SALTWATER Osmosis in a Plant Cell Lab Pre-LAB: 1. Summarize the information in the background 2. Define at least 4 terms in the introduction 3. Identify the purpose of the lab and record your hypothesis (prediction) 4. Write the procedure in your lab book PROCEDURE 1. Prepare a wet mount of red onion epidermis. Locate a good section of leaf under low power and then observe under high power. Sketch a single red onion cell in your lab book and describe the appearance of the plant cell. Label the cell wall, cell membrane and any other visible organelles. Use the colored pencils to color your diagram. 2. Add 2 or 3 drops of 15% NaCl to one edge of the cover slip. Draw the salt solution across the slide by touching a piece of paper towel to the fluid under the opposite edge of the cover slip. Observe the plant cells in the microscope while you draw the salt water across the slide. Sketch a single red onion cell in your lab book and describe what has happened to the plant cell. Label the cell wall, cell membrane and any other visible organelles. Use the colored pencils to color your diagram. 3. Flood the red onion tissue with fresh water using the same procedure you used for the salt water. Observe under high power and sketch a single red onion cell in your lab book. Describe and explain what has happened. Label the cell wall, cell membrane and any other visible organelles. Use the colored pencils to color your diagram. SUMMARY QUESTIONS 1. Describe what happens to the water content of the red onion cells when they are placed in a salt solution. Explain why this happened. 2. In the winter, icy roads are often salted to remove the ice and make them less slippery. Grasses and other herbaceous plants often die near the side of these roads. What causes this to happen? 3. When a person is given fluid intravenously (an I.V.) in the hospital, the fluid is typically a saline solution balanced — with the correct amount of dissolved materials — to human body tissues. Explain why this is necessary. 4. What if the unthinkable happened at the hospital! A patient was given an I.V. bag with distilled water in it rather than saline solution. Describe what would happen to their red blood cells and explain why this would occur. 5. Many freshwater one-celled organisms, like Paramecium, have contractile vacuoles. These structures collect and pump out excess water that accumulates in the cell. Explain why these organisms needs such a structure. 6. Explain why contractile vacuoles would be of no value to one-celled organisms living in salt water. 7. Popcorn sold at movie theatres is very salty, causing people to become thirsty and to buy soft drinks. Explain why salty popcorn causes this thirst. 8. Explain why soft-bodied invertebrates, like slugs, die when you pour salt on them. Conclusion: Write a conclusion paragraph. Refer to the rubric Osmosis in a Plant Cell Lab Checklist _____ (2)background information summary references osmosis in plant and animal cells. _____ (1)title is written at the top of the first page of the lab _____ (2)purpose of the lab is clearly stated, variables identified ( red onion cells and salt concentration) _____ (1)hypothesis is clearly stated, variables identified _____ (1)materials recorded _____ (3)procedure is clear and easy to follow _____ (2)initial red onion cell is clearly drawn, magnification is indicated _____ (2)initial red onion cell has cell wall and cell membrane labelled _____ (1)initial red onion cell is colored. _____ (1) description of initial _____ (2)salt water treated red onion cell is clearly drawn, magnification is indicated _____ (2)salt water treated red onion cell has cell wall and cell membrane labelled _____ (1)salt water treated red onion cell is colored. _____ (1) description of salt water treated cells _____ (2)distilled water treated red onion cell is clearly drawn, magnification is indicated _____ (2)distilled water treated red onion cell has cell wall and cell membrane labelled _____ (1)distilled water treated red onion cell is colored. _____ (1) description of distilled water treated cells. _____ (16)eight summary questions answered correctly (2 POINTS EACH) 1. ______ 2. _______ 3._______ 4. _______ 5. ______ 6. ______ 7. _____ 8.______ _____ (1)summary questions answered in complete sentences or questions written _____ (5)conclusion paragraph: (1pt each) Total points ________/ 50 possible VERIFICATION Graded By: (print name) __________________________________________ Date: _______________________ I verify that I assessed this lab report accurately and honestly, and only awarded points when clearly earned by the student whose lab book I was viewing. To the best of my knowledge, the score shown above is correct. I understand any points given in error can be taken from my lab points. Signed: ______________________________________________________